Question
A 103 molal aqueous solution of a chromium complex having same number of ammonia molecule and Cl ions freezes at -0.00558C. If all the
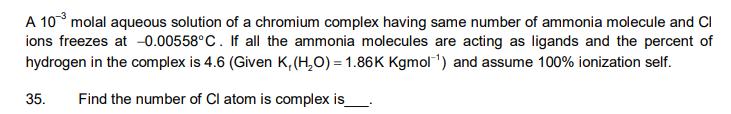
![]()
A 103 molal aqueous solution of a chromium complex having same number of ammonia molecule and Cl ions freezes at -0.00558C. If all the ammonia molecules are acting as ligands and the percent of hydrogen in the complex is 4.6 (Given K, (HO) = 1.86K Kgmol ) and assume 100% ionization self. 35. Find the number of Cl atom is complex is 36. The value of Vant Hoff factor is
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Chemistry Coursebook
Authors: Lawrie Ryan, Roger Norris
2nd Edition
1316637735, 978-1316637739
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



