Question
A 1.28-kg sample of water at 10.0C is in a calorimeter. You drop a piece of steel with a mass of 0.385 kg at
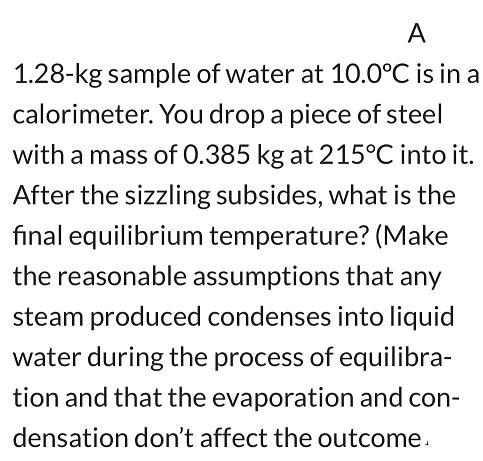
A 1.28-kg sample of water at 10.0C is in a calorimeter. You drop a piece of steel with a mass of 0.385 kg at 215C into it. After the sizzling subsides, what is the final equilibrium temperature? (Make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibra- tion and that the evaporation and con- densation don't affect the outcome.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the final equilibrium temperature when a piece of steel is dropped into water we can use the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



