Question
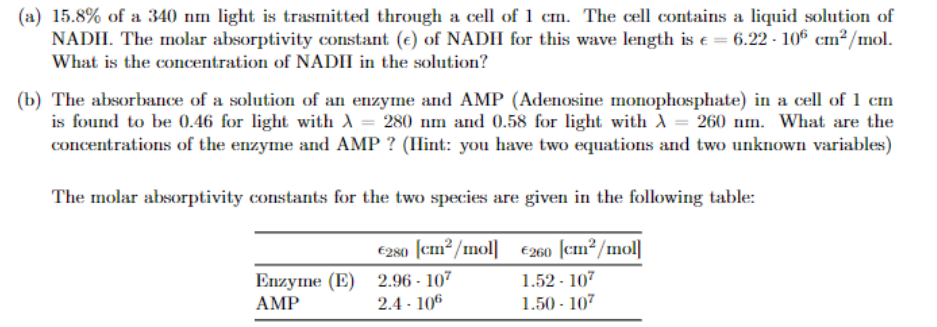
(a) 15.8% of a 340 nm light is trasmitted through a cell of 1 cm. The cell contains a liquid solution of NADH. The molar
(a) 15.8% of a 340 nm light is trasmitted through a cell of 1 cm. The cell contains a liquid solution of NADH. The molar absorptivity constant () of NADH for this wave length is = 6.22 106 cm2/mol. What is the concentration of NADH in the solution?
(b) The absorbance of a solution of an enzyme and AMP (Adenosine monophosphate) in a cell of 1 cm is found to be 0.46 for light with = 280 nm and 0.58 for light with = 260 nm. What are the concentrations of the enzyme and AMP ? (Hint: you have two equations and two unknown variables) The molar absorptivity constants for the two species are given in the following table:

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


