Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 18.6-g piece of gold (s ) = ( 0.129(J)/(gdeg C)) , initially at 285.4deg C , is add ded_(d) 147.2g of a liquid, initially
A 18.6-g piece of gold
(s)
=(
0.129(J)/(g\\\\deg C)), initially at
285.4\\\\deg C, is add
ded_(d)
147.2gof a liquid, initially at
27.9\\\\deg C, in an insulated container. The final temperature of the metal-liquid mixture at equilibrium is
28.9\\\\deg C. What is the identity of the liquid? Negle\ a. ethanol
(s)
=(
2.43(J)/(g*\\\\deg C))\ b. water
(s)
=(
4.53(J)/(g*\\\\deg C))\ d. acetone
(s)
=(
2.15(J)/(g*\\\\deg C))\ e. hexane
(s)
=(
2.27(J)/(g*\\\\deg C)) 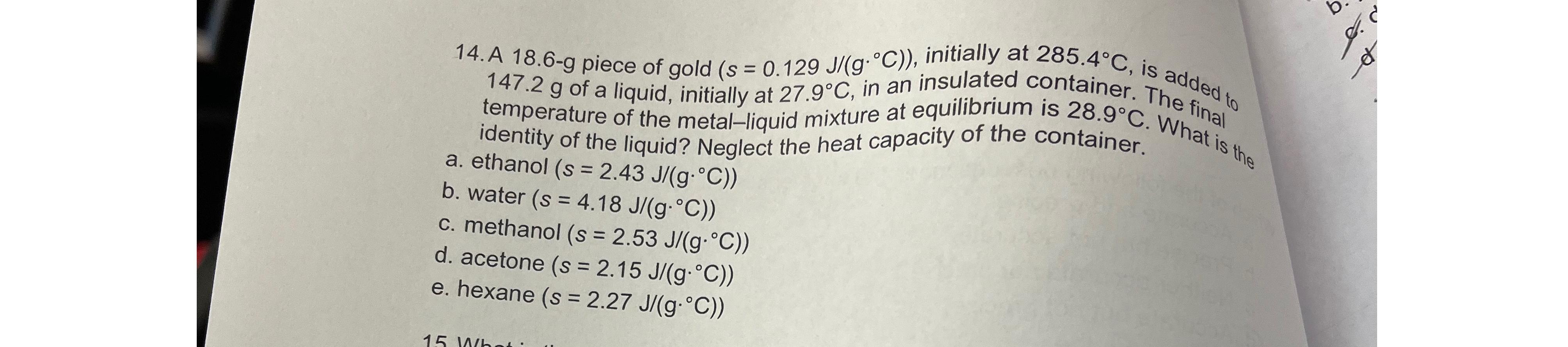
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


