Answered step by step
Verified Expert Solution
Question
1 Approved Answer
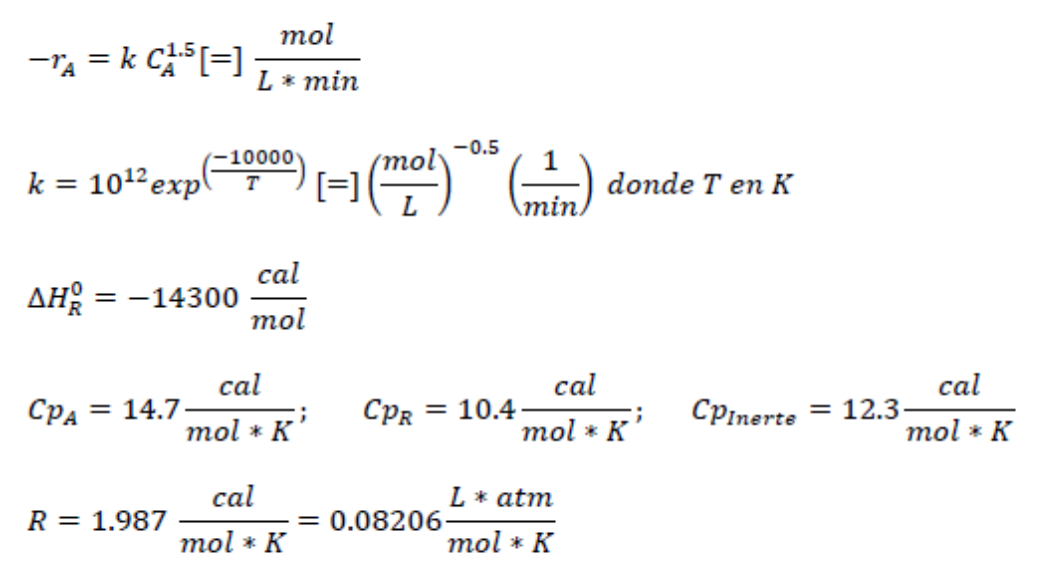
A - > 2 R The previous reaction is carried out in the gas phase within a CSTR reactor. The feed stream consists of 2
AR
The previous reaction is carried out in the gas phase within a CSTR reactor. The feed stream consists of Lmin of a mixture at deg C and atm whose composition is mol of A and mol of an inert gas, the conversion is desired to be
Additional Data:Consider that the values of the heat capacities are constant over the temperature range.
exp whereT
;;
FORM:
From the information provided, determine the value of the CA in molm From the information provided, determine the value of the initial concentration of inert CI in molm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


