Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 200 gm of oxygen gas has a volume 0.05 m at pressure 2 atm find its temperature according to Van der Waals' eqn
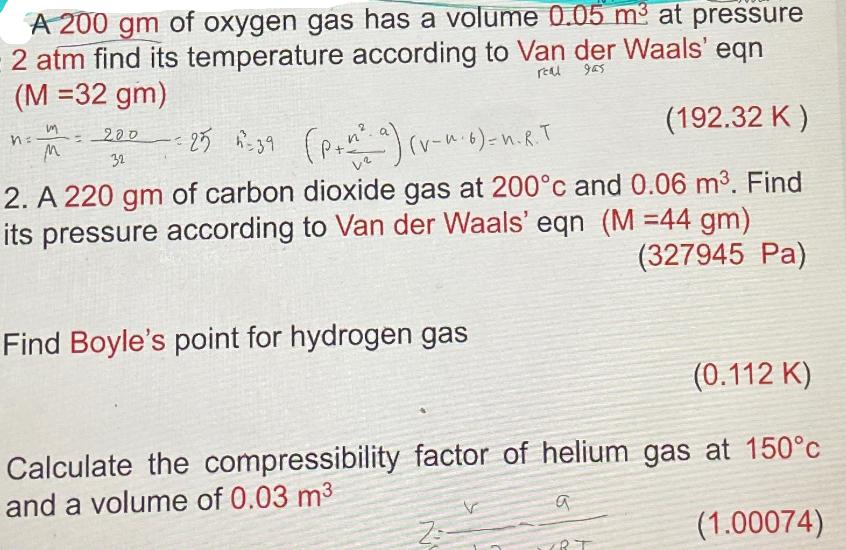
A 200 gm of oxygen gas has a volume 0.05 m at pressure 2 atm find its temperature according to Van der Waals' eqn (M =32 gm) real 965 (192.32 K) 2. A 220 gm of carbon dioxide gas at 200c and 0.06 m. Find its pressure according to Van der Waals' eqn (M =44 gm) (327945 Pa) m M = 200 32 C = 25 = 39 (p+n a) (v-^-6) = n. R. T Find Boyle's point for hydrogen gas (0.112 K) Calculate the compressibility factor of helium gas at 150c and a volume of 0.03 m (1.00074) a (BT
Step by Step Solution
There are 3 Steps involved in it
Step: 1
In the image there are two problems given that involve the Van der Waals equation which is a modified ideal gas law equation that accounts for the vol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


