Question
A 2.00-mol sample of hydrogen gas is heated at constant pressure from 290 K to 412 K. (a) Calculate the energy transferred to the
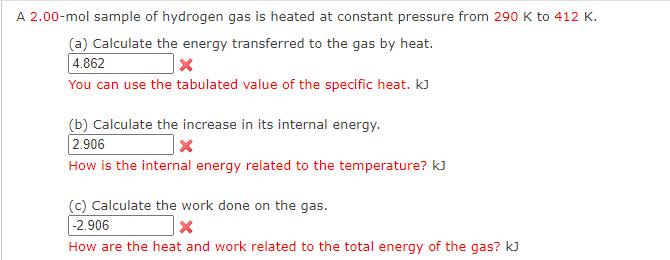
A 2.00-mol sample of hydrogen gas is heated at constant pressure from 290 K to 412 K. (a) Calculate the energy transferred to the gas by heat. 4.862 You can use the tabulated value of the specific heat. KJ (b) Calculate the increase in its internal energy. 2.906 How is the internal energy related to the temperature? kJ (c) Calculate the work done on the gas. -2.906 How are the heat and work related to the total energy of the gas? KJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a To calculate the energy transferred to the gas by heat you can use the formula Q n C T where Q hea...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



