
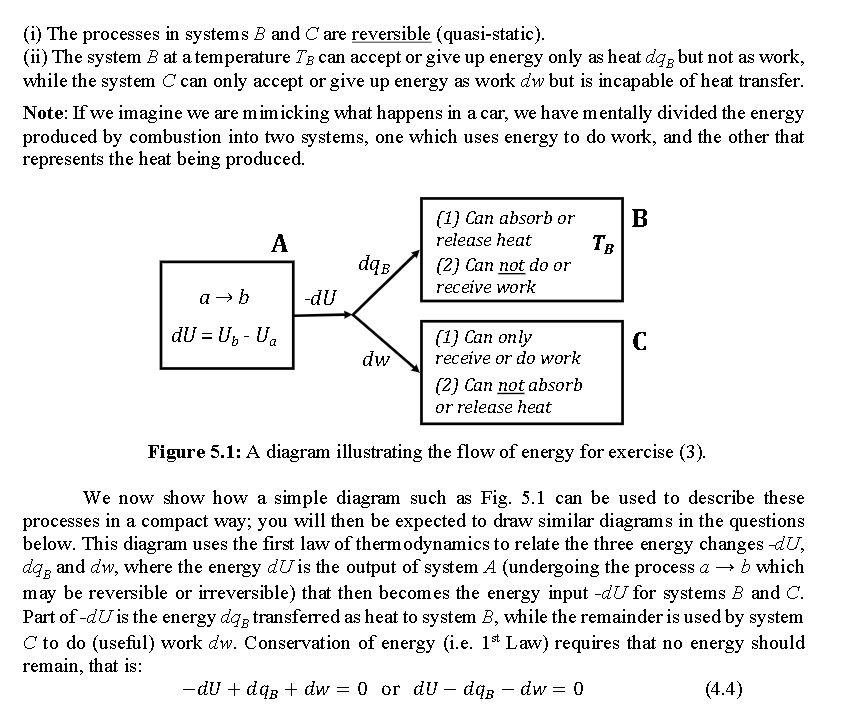

a - (3) The thermodynamic driving force causing systems to undergo change can be channelled to do useful work. In this question we examine the way in which maximum work output from a system undergoing a process a + b can be achieved. As we have already seen, during a process the values of the parameters characterizing the state a change to those that characterize the state b. In this case Ay, which represents the change in the parameter y, will be equal to the value of y in state b minus its value in state a, and includes the sign of the change. If the change is infinitesimally small, it is designated by dy. As an example for discussion, consider the reaction: (g(- CH4(8) + 2 O2(g) CO2(g) + 2 H20 (8) b Here the state a is the fuel mixture of methane and oxygen in an engine, while the state b is the combustion product mixture. In an engine this reaction produces energy (that can be used) because the energy Us of the products is less than the energy Ua of the reactants. This means that: AU= U, -Ua is negative. Now if another part of the machine is going to make use of this energy, it will be receiving -AU from the engine. It is important to fully appreciate the signs of these changes. If a system produces a quantity 4y as an output and another system receives this quantity as input then the latter receives -Ay. We know from everyday experience that whenever we run a machine (such as a car) not only do we get useful work but heat is also produced. Thus, the energy being produced is being partitioned into two parts: (1) useful work; and (2) heat. For efficient energy (and hence fuel) usage we would like to maximize the work component and minimize the heat component. If we consider the processes to be reversible (i.e. quasi-static), then the heat component is related to entropy change by a relationship dgrev = Tds. The problem of efficient energy usage will now be the theme of the questions below. Consider a system A that undergoes the process ab and experiences a change in energy given by dU; as a consequence, energy-dUbecomes available for use (note here we have switched to considering infinitesimal changes). Let us take this energy and transfer it to two other systems B and C (see Fig. 5.1) which are both characterized by the following properties: (i) The processes in systems B and C are reversible (quasi-static). (ii) The system B at a temperature Te can accept or give up energy only as heat dag but not as work, while the system C can only accept or give up energy as work dw but is incapable of heat transfer. Note: If we imagine we are mimicking what happens in a car, we have mentally divided the energy produced by combustion into two systems, one which uses energy to do work, and the other that represents the heat being produced. B A (1) Can absorb or release heat (2) Can not do or receive work dqB a -dU dU = Un-U C dw (1) Can only receive or do work (2) Can not absorb or release heat Figure 5.1: A diagram illustrating the flow of energy for exercise (3). We now show how a simple diagram such as Fig. 5.1 can be used to describe these processes in a compact way; you will then be expected to draw similar diagrams in the questions below. This diagram uses the first law of thermodynamics to relate the three energy changes du, dog and dw, where the energy dU is the output of system A (undergoing the process a + b which may be reversible or irreversible) that then becomes the energy input -dU for systems B and C. Part of -dU is the energy dag transferred as heat to system B, while the remainder is used by system C to do (useful) work dw. Conservation of energy (i.e. 1st Law) requires that no energy should remain, that is: -du + dos + dw = 0 or du db - dw=0 (4.4) (a) In our example above, some of dU (the energy release in A) could be used to change entropy. Let this entropy change be ds, and the total change in entropy for the combined systems (A + B+C) be dStor. Provide expressions for dsg and dSc. Then give an equation for dStor in terms of the entropy changes of the constituent systems. Are there any restrictions on the possible values of dStot, and if yes what are they? Remember: (1) that the systems B and C are undergoing reversible processes; (2) the process a + b in system A may be reversible or irreversible; (3) the relationship between heat and entropy changes for reversible processes. (b) Use the result obtained in (a) and solve for deg. Then insert this result into the equation (4.4) and derive an equation for the work dw that can be obtained from the energy release dU. Then show that the maximum work is obtained if and only if the entire process involving the systems A, B and C is carried out reversibly, that is dStor=0. This is a very important result and is often called the Maximum Work Theorem (also recall your results from lab 1). There is a practical side to this analysis which we study in the next exercise. a - (3) The thermodynamic driving force causing systems to undergo change can be channelled to do useful work. In this question we examine the way in which maximum work output from a system undergoing a process a + b can be achieved. As we have already seen, during a process the values of the parameters characterizing the state a change to those that characterize the state b. In this case Ay, which represents the change in the parameter y, will be equal to the value of y in state b minus its value in state a, and includes the sign of the change. If the change is infinitesimally small, it is designated by dy. As an example for discussion, consider the reaction: (g(- CH4(8) + 2 O2(g) CO2(g) + 2 H20 (8) b Here the state a is the fuel mixture of methane and oxygen in an engine, while the state b is the combustion product mixture. In an engine this reaction produces energy (that can be used) because the energy Us of the products is less than the energy Ua of the reactants. This means that: AU= U, -Ua is negative. Now if another part of the machine is going to make use of this energy, it will be receiving -AU from the engine. It is important to fully appreciate the signs of these changes. If a system produces a quantity 4y as an output and another system receives this quantity as input then the latter receives -Ay. We know from everyday experience that whenever we run a machine (such as a car) not only do we get useful work but heat is also produced. Thus, the energy being produced is being partitioned into two parts: (1) useful work; and (2) heat. For efficient energy (and hence fuel) usage we would like to maximize the work component and minimize the heat component. If we consider the processes to be reversible (i.e. quasi-static), then the heat component is related to entropy change by a relationship dgrev = Tds. The problem of efficient energy usage will now be the theme of the questions below. Consider a system A that undergoes the process ab and experiences a change in energy given by dU; as a consequence, energy-dUbecomes available for use (note here we have switched to considering infinitesimal changes). Let us take this energy and transfer it to two other systems B and C (see Fig. 5.1) which are both characterized by the following properties: (i) The processes in systems B and C are reversible (quasi-static). (ii) The system B at a temperature Te can accept or give up energy only as heat dag but not as work, while the system C can only accept or give up energy as work dw but is incapable of heat transfer. Note: If we imagine we are mimicking what happens in a car, we have mentally divided the energy produced by combustion into two systems, one which uses energy to do work, and the other that represents the heat being produced. B A (1) Can absorb or release heat (2) Can not do or receive work dqB a -dU dU = Un-U C dw (1) Can only receive or do work (2) Can not absorb or release heat Figure 5.1: A diagram illustrating the flow of energy for exercise (3). We now show how a simple diagram such as Fig. 5.1 can be used to describe these processes in a compact way; you will then be expected to draw similar diagrams in the questions below. This diagram uses the first law of thermodynamics to relate the three energy changes du, dog and dw, where the energy dU is the output of system A (undergoing the process a + b which may be reversible or irreversible) that then becomes the energy input -dU for systems B and C. Part of -dU is the energy dag transferred as heat to system B, while the remainder is used by system C to do (useful) work dw. Conservation of energy (i.e. 1st Law) requires that no energy should remain, that is: -du + dos + dw = 0 or du db - dw=0 (4.4) (a) In our example above, some of dU (the energy release in A) could be used to change entropy. Let this entropy change be ds, and the total change in entropy for the combined systems (A + B+C) be dStor. Provide expressions for dsg and dSc. Then give an equation for dStor in terms of the entropy changes of the constituent systems. Are there any restrictions on the possible values of dStot, and if yes what are they? Remember: (1) that the systems B and C are undergoing reversible processes; (2) the process a + b in system A may be reversible or irreversible; (3) the relationship between heat and entropy changes for reversible processes. (b) Use the result obtained in (a) and solve for deg. Then insert this result into the equation (4.4) and derive an equation for the work dw that can be obtained from the energy release dU. Then show that the maximum work is obtained if and only if the entire process involving the systems A, B and C is carried out reversibly, that is dStor=0. This is a very important result and is often called the Maximum Work Theorem (also recall your results from lab 1). There is a practical side to this analysis which we study in the next exercise









