Question
A 35% solution of Na2CO3 weighing 6000 kg is cooled to 20C to yield crystals of Na2CO3.10 HO. During cooling 4% by weight of
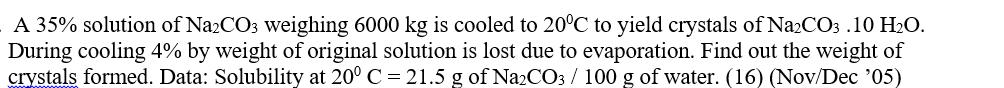
A 35% solution of Na2CO3 weighing 6000 kg is cooled to 20C to yield crystals of Na2CO3.10 HO. During cooling 4% by weight of original solution is lost due to evaporation. Find out the weight of crystals formed. Data: Solubility at 20 C = 21.5 g of Na2CO3 / 100 g of water. (16) (Nov/Dec '05)
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
We can start by using the information provided to find out the weight of water evaporated ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
9th edition
978-1118546895, 111854689X, 978-1118477700, 1118477707, 1118324579, 978-1118324578
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



