Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 46.91 mL solution of weak acid is titrated with 0.429 M NaOH. It requires 34.58 mL of the NaOH titrant to reach the
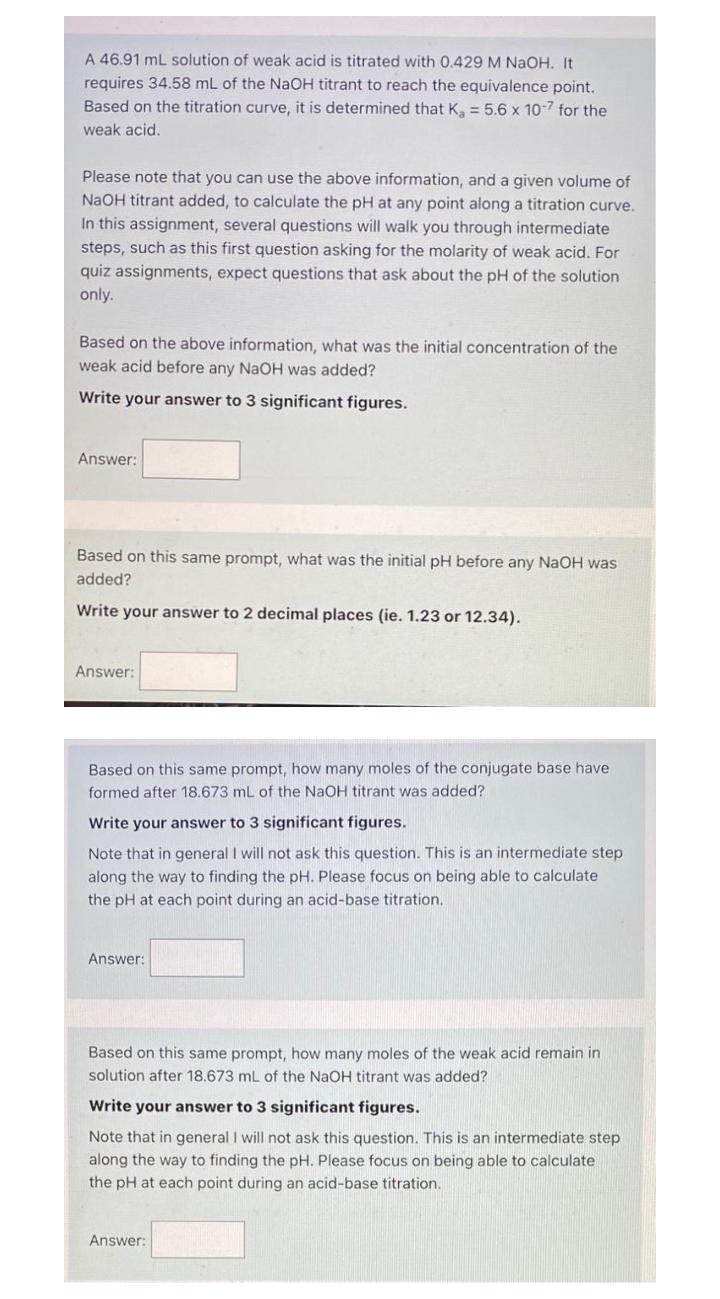
A 46.91 mL solution of weak acid is titrated with 0.429 M NaOH. It requires 34.58 mL of the NaOH titrant to reach the equivalence point. Based on the titration curve, it is determined that K = 5.6 x 10-7 for the weak acid. Please note that you can use the above information, and a given volume of NaOH titrant added, to calculate the pH at any point along a titration curve. In this assignment, several questions will walk you through intermediate steps, such as this first question asking for the molarity of weak acid. For quiz assignments, expect questions that ask about the pH of the solution only. Based on the above information, what was the initial concentration of the weak acid before any NaOH was added? Write your answer to 3 significant figures. Answer: Based on this same prompt, what was the initial pH before any NaOH was added? Write your answer to 2 decimal places (ie. 1.23 or 12.34). Answer: Based on this same prompt, how many moles of the conjugate base have formed after 18.673 mL of the NaOH titrant was added? Write your answer to 3 significant figures. Note that in general I will not ask this question. This is an intermediate step along the way to finding the pH. Please focus on being able to calculate the pH at each point during an acid-base titration. Answer: Based on this same prompt, how many moles of the weak acid remain in solution after 18.673 mL of the NaOH titrant was added? Write your answer to 3 significant figures. Note that in general I will not ask this question. This is an intermediate step along the way to finding the pH. Please focus on being able to calculate the pH at each point during an acid-base titration. Answer:
Step by Step Solution
★★★★★
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


