Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 4.76 sample of a solid containing Ni is dissolved in 20.0 mL water.. Determine the molar concentration of Ni in the 20.0 mL solution.
A 4.76 sample of a solid containing Ni is dissolved in 20.0 mL water.. Determine the molar concentration of Ni in the 20.0 mL solution. 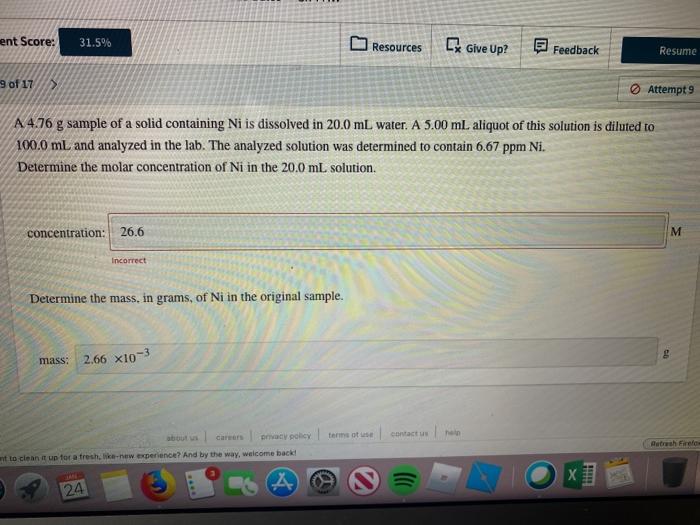
ent Score: 31.5% Resources Ex Give Up? Feedback Resume 9 of 17 > Attempt 9 A 4.76 g sample of a solid containing Ni is dissolved in 20.0 mL water. A 5.00 mL aliquot of this solution is diluted to 100.0 mL and analyzed in the lab. The analyzed solution was determined to contain 6.67 ppm Ni. Determine the molar concentration of Ni in the 20.0 mL solution. concentration: 26.6 M incorrect Determine the mass, in grams, of Ni in the original sample. mass: 2.66 X10-3 boutis laevacy policy terms of use contact us ant to clean it up for a fresh new experience? And by the way, welcome back! X 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


