Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a 5 Question (2 points) The gas-phase decomposition of ethyl bromide is a first-order reaction, occurring with a rate constant that demonstrates the following dependence
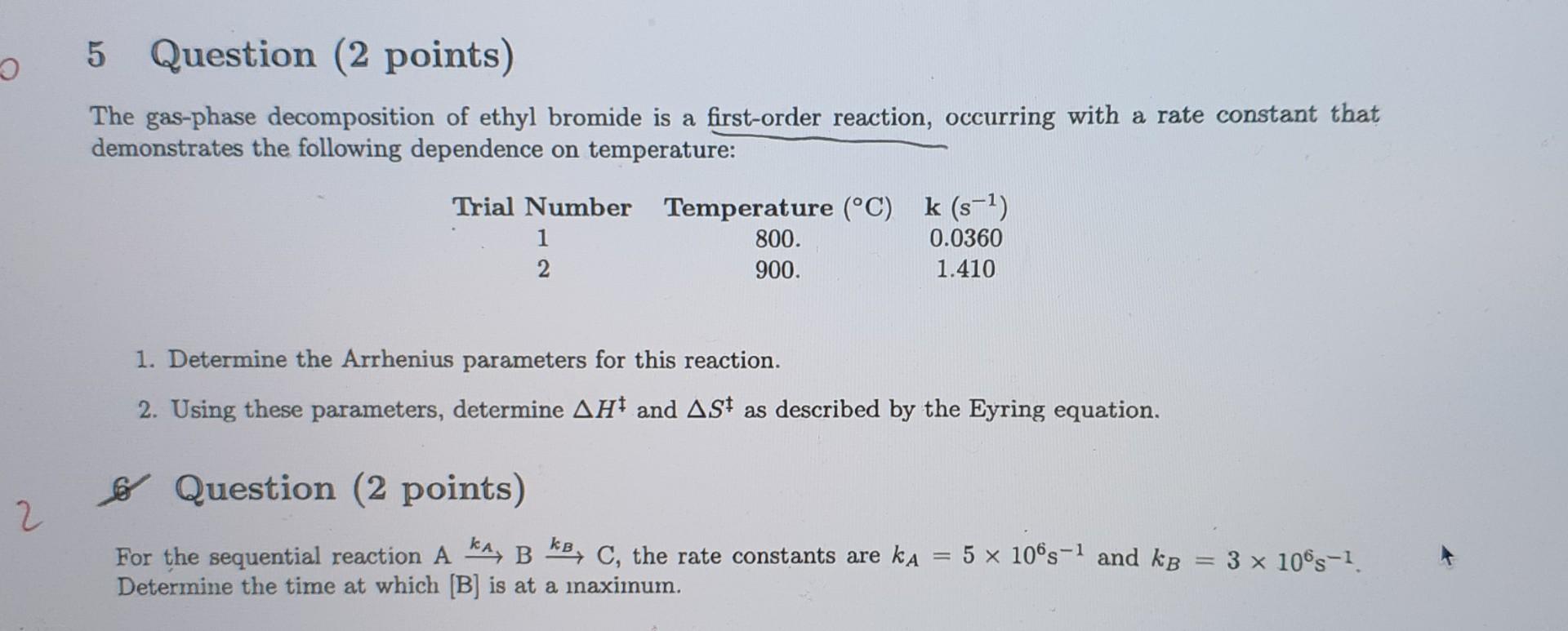
a 5 Question (2 points) The gas-phase decomposition of ethyl bromide is a first-order reaction, occurring with a rate constant that demonstrates the following dependence on temperature: Trial Number Temperature (C) k (s-1) 1 800. 0.0360 2 900. 1.410 1. Determine the Arrhenius parameters for this reaction. 2. Using these parameters, determine AH and ASt as described by the Eyring equation. Lo Question (2 points) 2 5 x 10s-1 and kb For the sequential reaction A kA, B kB, C, the rate constants are ka Determine the time at which [B] is at a maximum. 3 x 10s-1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


