Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 50 wt % hydrochloride acid solution [HCl(aq)] is produced by using water to absorb gaseous hydrogen chloride [HCl(g)] in an absorption column. Liquid water
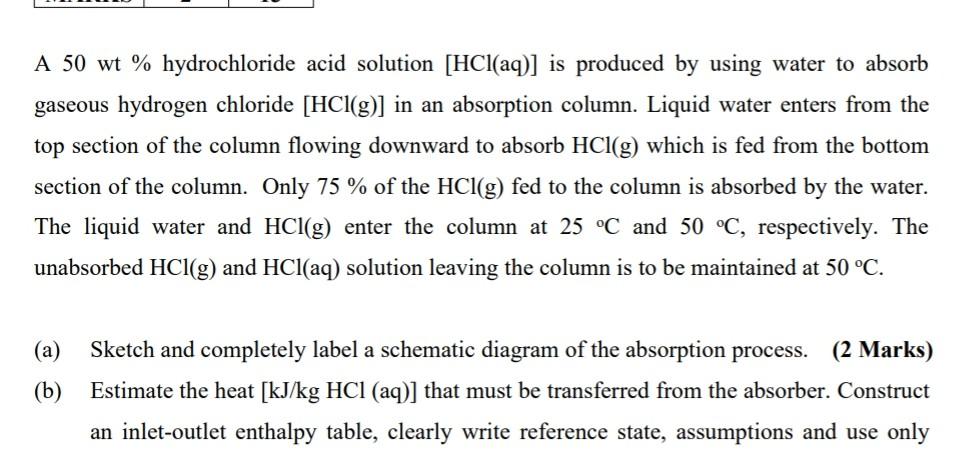
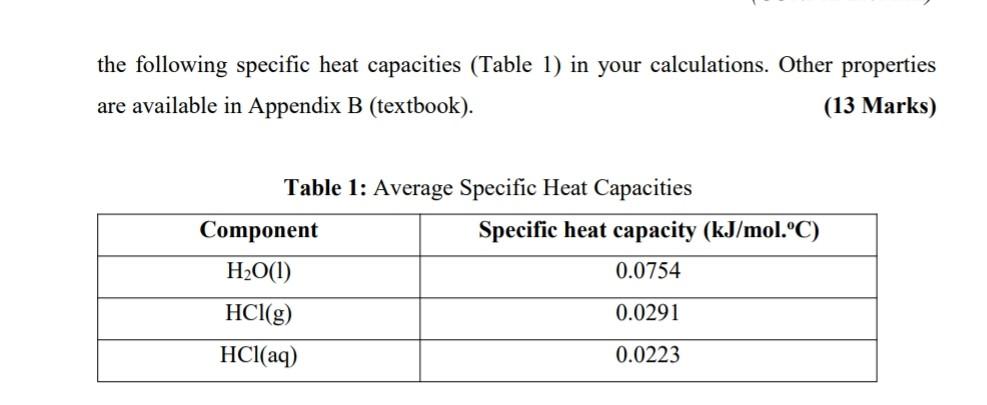
A 50 wt % hydrochloride acid solution [HCl(aq)] is produced by using water to absorb gaseous hydrogen chloride [HCl(g)] in an absorption column. Liquid water enters from the top section of the column flowing downward to absorb HCl(g) which is fed from the bottom section of the column. Only 75 % of the HCl(g) fed to the column is absorbed by the water. The liquid water and HCl(g) enter the column at 25 C and 50 C, respectively. The unabsorbed HCl(g) and HCl(aq) solution leaving the column is to be maintained at 50 C. (a) Sketch and completely label a schematic diagram of the absorption process. (2 Marks) (b) Estimate the heat [kJ/kg HCl (aq)] that must be transferred from the absorber. Construct an inlet-outlet enthalpy table, clearly write reference state, assumptions and use only the following specific heat capacities (Table 1) in your calculations. Other properties are available in Appendix B (textbook). (13 Marks) Table 1: Average Specific Heat Capacities Component Specific heat capacity (kJ/mol.C) H2O(1) 0.0754 HCl(g) 0.0291 HCl(aq) 0.0223
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


