Question
A 6.0 g ice cube at -10C is in a rigid, sealed container from which all the air has been evacuated (which means you
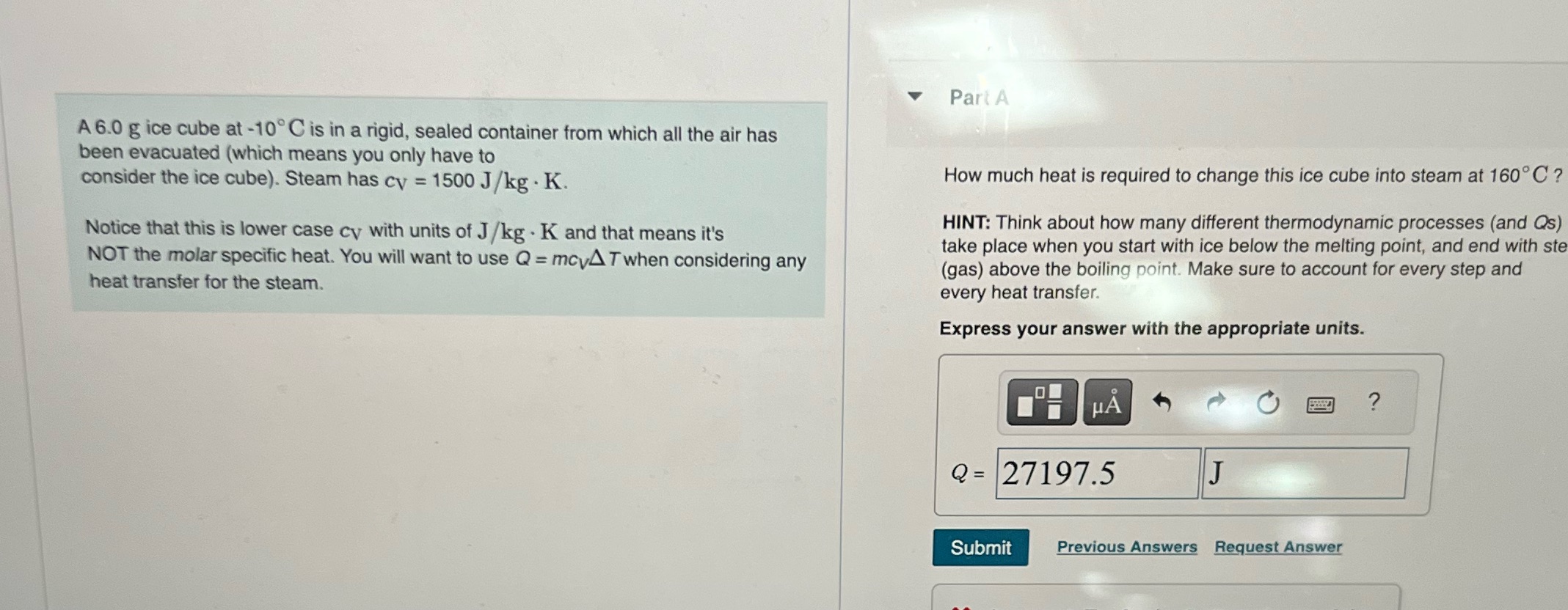
A 6.0 g ice cube at -10C is in a rigid, sealed container from which all the air has been evacuated (which means you only have to consider the ice cube). Steam has cy = 1500 J/kg. K. . Notice that this is lower case cy with units of J/kg K and that means it's NOT the molar specific heat. You will want to use Q = mcyAT when considering any heat transfer for the steam. Part A How much heat is required to change this ice cube into steam at 160C? HINT: Think about how many different thermodynamic processes (and Qs) take place when you start with ice below the melting point, and end with ste (gas) above the boiling point. Make sure to account for every step and every heat transfer. Express your answer with the appropriate units. H Q=27197.5 J Submit Previous Answers Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics for Scientists and Engineers A Strategic Approach with Modern Physics
Authors: Randall D. Knight
4th edition
978-0134092508, 134092503, 133942651, 978-0133942651
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



