Question
A 64.3 mg sample of a protein (MW = 58,600) was treated with 2.00 mL of 0.0487 M sodium periodate (NaIO4) to selectively react
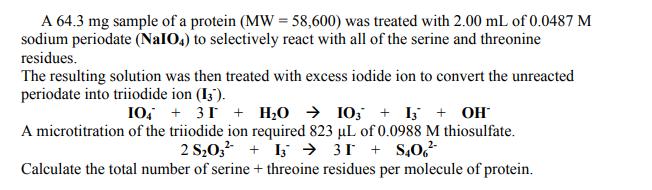
A 64.3 mg sample of a protein (MW = 58,600) was treated with 2.00 mL of 0.0487 M sodium periodate (NaIO4) to selectively react with all of the serine and threonine residues. The resulting solution was then treated with excess iodide ion to convert the unreacted periodate into triiodide ion (13). 10 +31 + HO 103 + 13 + OH A microtitration of the triiodide ion required 823 L of 0.0988 M thiosulfate. 2 S031331+S406 Calculate the total number of serine + threoine residues per molecule of protein.
Step by Step Solution
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



