Question
A 6-L ideal gas with y = 1.5 is at 300 K and 100 kPa. The gas is compressed adiabatically until its volume becomes
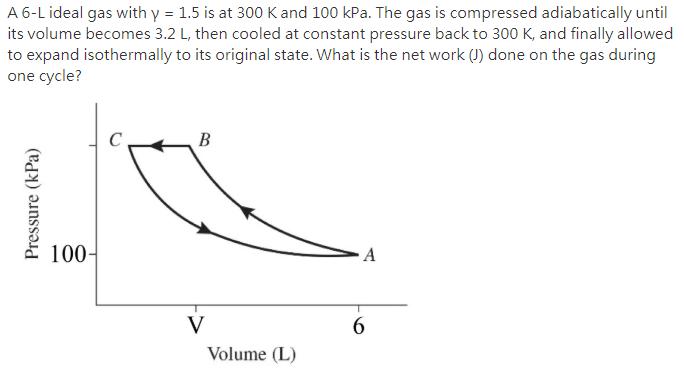
A 6-L ideal gas with y = 1.5 is at 300 K and 100 kPa. The gas is compressed adiabatically until its volume becomes 3.2 L, then cooled at constant pressure back to 300 K, and finally allowed to expand isothermally to its original state. What is the net work (J) done on the gas during one cycle? Pressure (kPa) 100- C B V Volume (L) A 6
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the net work done on the gas during one cycle we need to calculate the work done in each sta...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



