Answered step by step
Verified Expert Solution
Question
1 Approved Answer
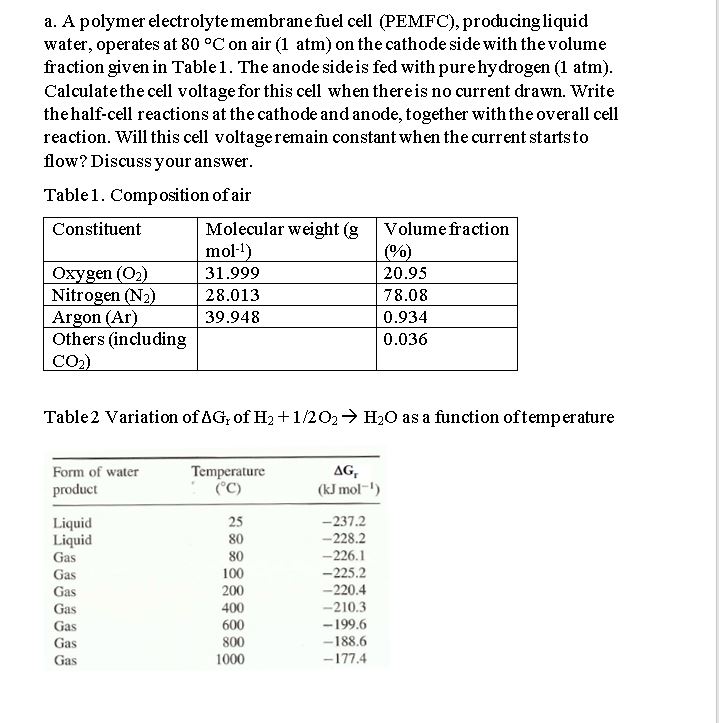
a . A polymer electrolyte membrane fuel cell ( PEMFC ) , producing liquid water, operates at 8 0 C on air ( 1 atm
a A polymer electrolyte membrane fuel cell PEMFC producing liquid
water, operates at on air atm on the cathode side with the volume
fraction given in Table The anode side is fed with pure hydrogen atm
Calculate the cell voltage for this cell when there is no current drawn. Write
the halfcell reactions at the cathode and anode, together with the overall cell
reaction. Will this cell voltage remain constant when the current starts to
flow? Discuss your answer.
Table Composition of air
Table Variation of of as a function of temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


