Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) and b) please (b) Find [Na]. 5-25. Europium is a lanthanide element found at parts per billion levels in natural waters. It can be
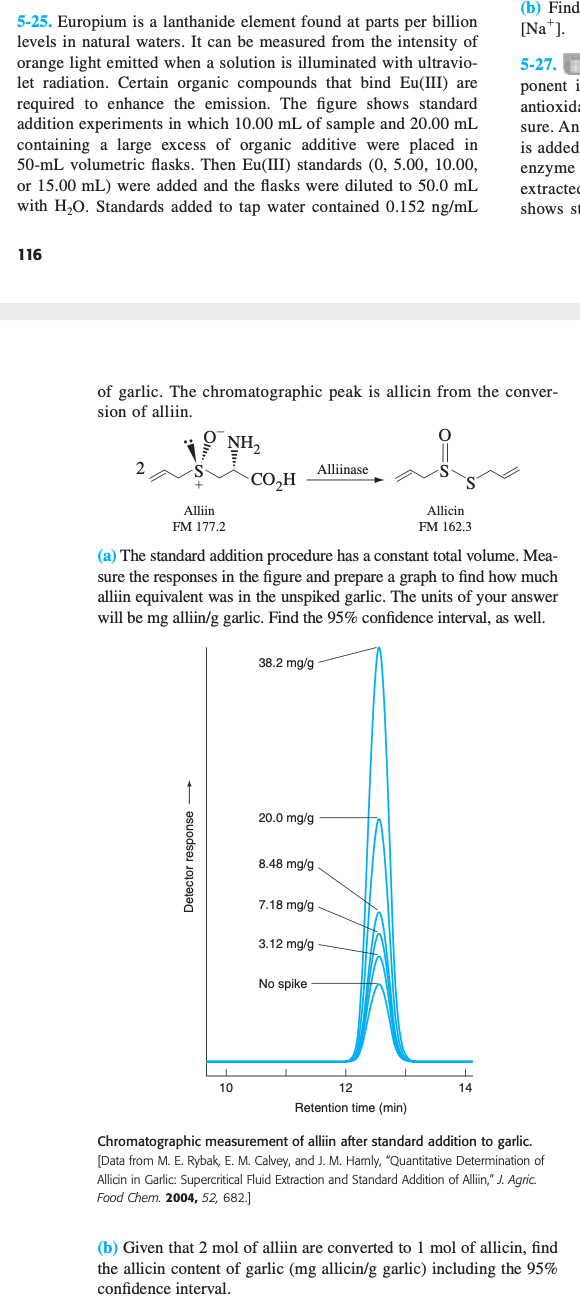
a) and b) please
(b) Find [Na]. 5-25. Europium is a lanthanide element found at parts per billion levels in natural waters. It can be measured from the intensity of orange light emitted when a solution is illuminated with ultravio- let radiation. Certain organic compounds that bind Eu(III) are required to enhance the emission. The figure shows standard addition experiments in which 10.00 mL of sample and 20.00 mL containing a large excess of organic additive were placed in 50-mL volumetric flasks. Then Eu(III) standards (0, 5.00, 10.00, or 15.00 mL) were added and the flasks were diluted to 50.0 mL with H20. Standards added to tap water contained 0.152 ng/mL 5-27. ponent i antioxida sure. An is added enzyme extracted shows st 116 of garlic. The chromatographic peak is allicin from the conver- sion of alliin. 0 NH 2 Alliinase COH Alliin FM 177.2 Allicin FM 162.3 (a) The standard addition procedure has a constant total volume. Mea- sure the responses in the figure and prepare a graph to find how much alliin equivalent was in the unspiked garlic. The units of your answer will be mg alliin/g garlic. Find the 95% confidence interval, as well. 38.2 mg/g 20.0 mg/g Detector response 8.48 mg/g 7.18 mg/g 3.12 mg/g No spike 10 14 12 Retention time (min) Chromatographic measurement of alliin after standard addition to garlic. [Data from M. E. Rybak, E. M. Calvey, and J. M. Harnly, "Quantitative Determination of du Allicin in Garlic: Supercritical Fluid Extraction and Standard Addition of Alliin," J. Agric Food Chem. 2004, 52, 682.] (b) Given that 2 mol of alliin are converted to 1 mol of allicin, find the allicin content of garlic (mg allicin/g garlic) including the 95% confidence intervalStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


