Answered step by step
Verified Expert Solution
Question
1 Approved Answer
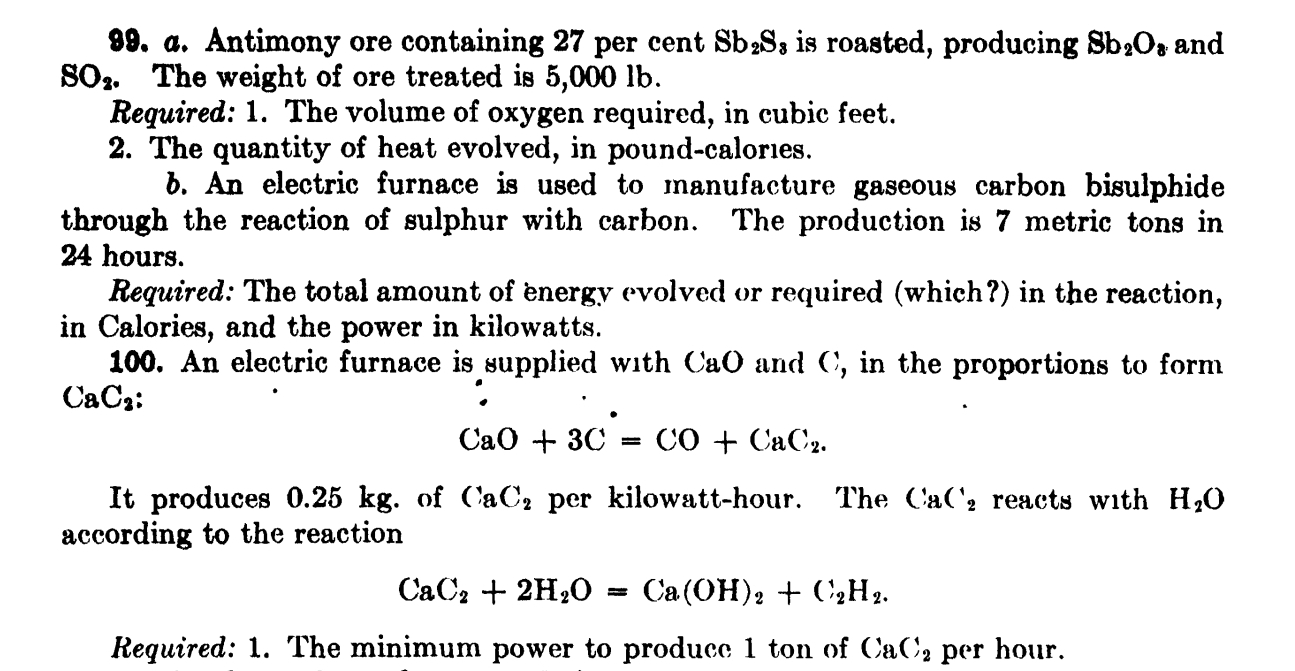
a . Antimony ore containing 2 7 per cent S b 2 S 8 is roasted, producing S b 2 O 3 and S O
a Antimony ore containing per cent is roasted, producing and The weight of ore treated is
Required: The volume of oxygen required, in cubic feet.
The quantity of heat evolved, in poundcalories.
b An electric furnace is used to manufacture gaseous carbon bisulphide through the reaction of sulphur with carbon. The production is metric tons in hours.
Required: The total amount of energy evolved or required which in the reaction, in Calories, and the power in kilowatts.
An electric furnace is supplied with CaO and in the proportions to form :
@
It produces of per kilowatthour. The reacts with according to the reaction
Required: The minimum power to produce ton of per hour.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


