Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) Apply the CSTR design equation at 450K continue with Arrhenius equation to find k at 460K use Thiele modulus to find n460K and

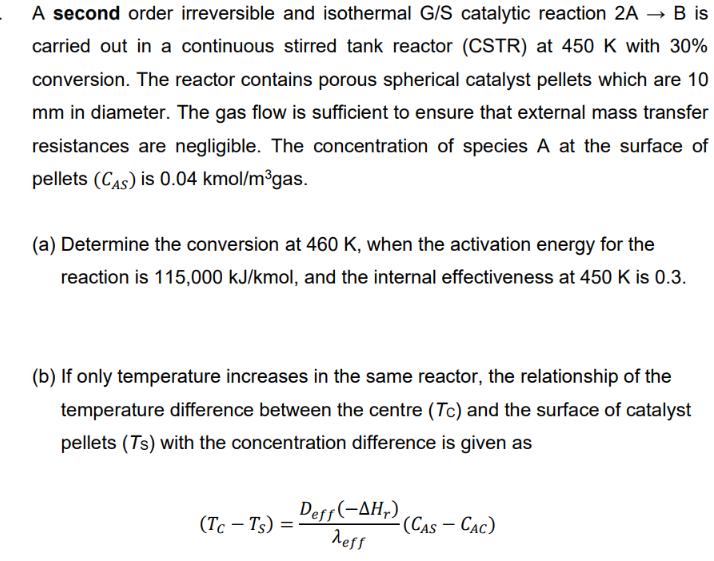

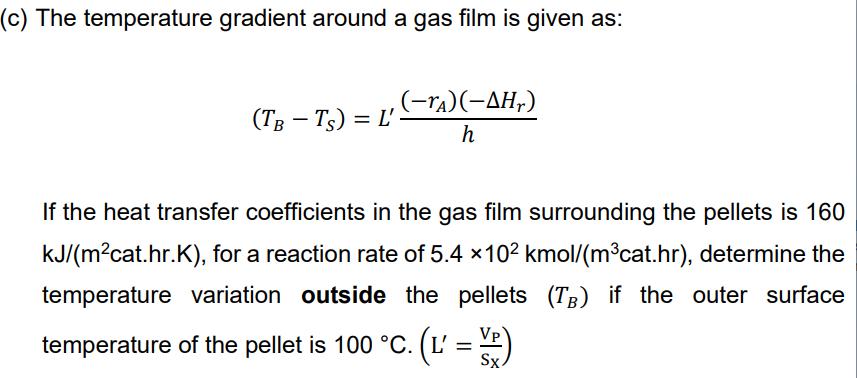
a) Apply the CSTR design equation at 450K continue with Arrhenius equation to find k at 460K use Thiele modulus to find n460K and then X. (X=0.368) b) maximum temperature in pellet takes place when CAC=0; (Tc=121.4 C) c) at steady state apply Qgen = Qrem; (TB=102.4 C) A second order irreversible and isothermal G/S catalytic reaction 2A B is carried out in a continuous stirred tank reactor (CSTR) at 450 K with 30% conversion. The reactor contains porous spherical catalyst pellets which are 10 mm in diameter. The gas flow is sufficient to ensure that external mass transfer resistances are negligible. The concentration of species A at the surface of pellets (CAS) is 0.04 kmol/mgas. (a) Determine the conversion at 460 K, when the activation energy for the reaction is 115,000 kJ/kmol, and the internal effectiveness at 450 K is 0.3. (b) If only temperature increases in the same reactor, the relationship of the temperature difference between the centre (Tc) and the surface of catalyst pellets (Ts) with the concentration difference is given as (Tc-Ts) = Deff(-AHr) eff -(CAS - CAC) where CAC is the concentration at the center of the pellets, and (-AH,) is 4.8x104 kJ/kmol and (Aeff) is 2.610-4 kJ/(m cat C.s). Calculate the temperature variation within the pellet if the outer surface temperature of the pellet is now 100 C and effective diffusivity of A in the porous pellets is 2.9 x 10-6 mgas/(mcat s). (c) The temperature gradient around a gas film is given as: (TB-Ts) = L' (-ra)(-,) h If the heat transfer coefficients in the gas film surrounding the pellets is 160 kJ/(mcat.hr.K), for a reaction rate of 5.4 x 10 kmol/(mcat.hr), determine the temperature variation outside the pellets (TB) if the outer surface temperature of the pellet is 100 C. (L' = VR) Sx.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


