Question: A. Assume propane at this state can be described by ideal gas, and determine the density of propane at 300 K and 800 kPa.
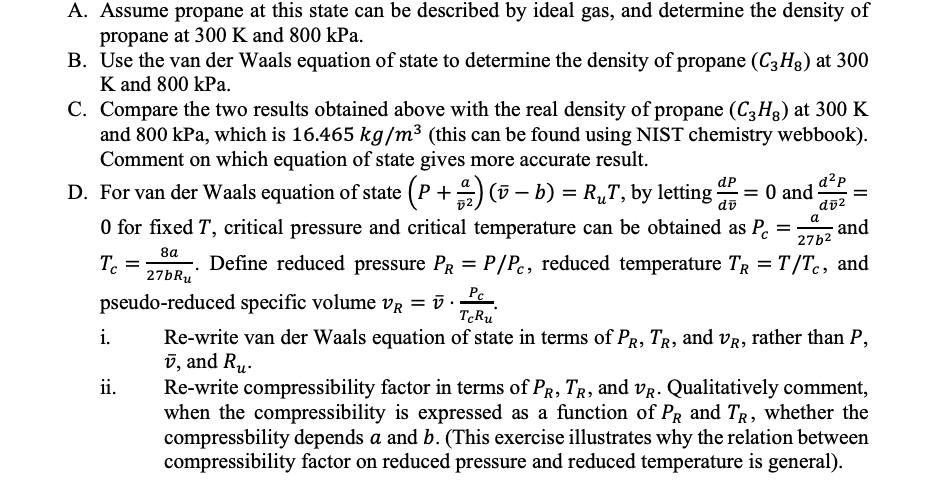
A. Assume propane at this state can be described by ideal gas, and determine the density of propane at 300 K and 800 kPa. B. Use the van der Waals equation of state to determine the density of propane (C3H8) at 300 K and 800 kPa. C. Compare the two results obtained above with the real density of propane (C3H) at 300 K and 800 kPa, which is 16.465 kg/m (this can be found using NIST chemistry webbook). Comment on which equation of state gives more accurate result. D. For van der Waals equation of state (P + )(v b) = RT, by letting d = 0 and dP dv 0 for fixed T, critical pressure and critical temperature can be obtained as P a = 8a 27b Tc i. dp dv -. Define reduced pressure PR = P/Pc, reduced temperature TR = T/Tc, and 27bRu pseudo-reduced specific volume VR = v. TcRu ii. = = and Re-write van der Waals equation of state in terms of PR, TR, and VR, rather than P, v, and Ru Re-write compressibility factor in terms of PR, TR, and VR. Qualitatively comment, when the compressibility is expressed as a function of PR and TR, whether the compressbility depends a and b. (This exercise illustrates why the relation between compressibility factor on reduced pressure and reduced temperature is general).
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


