Answered step by step
Verified Expert Solution
Question
1 Approved Answer
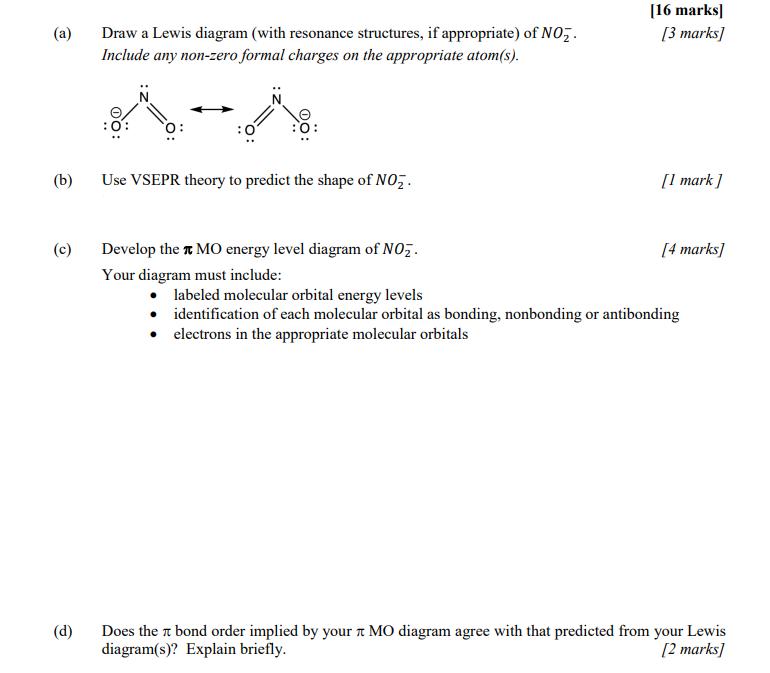
(a) (b) (c) (d) Draw a Lewis diagram (with resonance structures, if appropriate) of NO. Include any non-zero formal charges on the appropriate atom(s).
![]()
(a) (b) (c) (d) Draw a Lewis diagram (with resonance structures, if appropriate) of NO. Include any non-zero formal charges on the appropriate atom(s). Use VSEPR theory to predict the shape of NO. [16 marks] [3 marks] [1 mark] Develop the MO energy level diagram of NO. Your diagram must include: labeled molecular orbital energy levels identification of each molecular orbital as bonding, nonbonding or antibonding electrons in the appropriate molecular orbitals [4 marks] Does the bond order implied by your MO diagram agree with that predicted from your Lewis diagram(s)? Explain briefly. [2 marks] (e) Draw the MOS. Show both a top view and a side view for each MO. Label each picture so that it is clear which & MO it shows. [6 marks]
Step by Step Solution
★★★★★
3.41 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


