Answered step by step
Verified Expert Solution
Question
1 Approved Answer
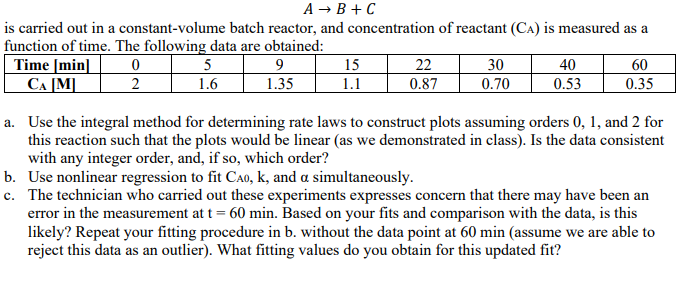
A B + C is carried out in a constant - volume batch reactor, and concentration of reactant ( C A A ) is measured
is carried out in a constantvolume batch reactor, and concentration of reactant is measured as a
function of time. The following data are obtained:
a Use the integral method for determining rate laws to construct plots assuming orders and for
this reaction such that the plots would be linear as we demonstrated in class Is the data consistent
with any integer order, and, if so which order?
b Use nonlinear regression to fit and simultaneously.
c The technician who carried out these experiments expresses concern that there may have been an
error in the measurement at min. Based on your fits and comparison with the data, is this
likely? Repeat your fitting procedure in b without the data point at min assume we are able to
reject this data as an outlier What fitting values do you obtain for this updated fit?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


