Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) (b) In 1956 a breakdown of the Rutherford formula for the differential scattering cross section was observed experimentally by Farewell et al. In
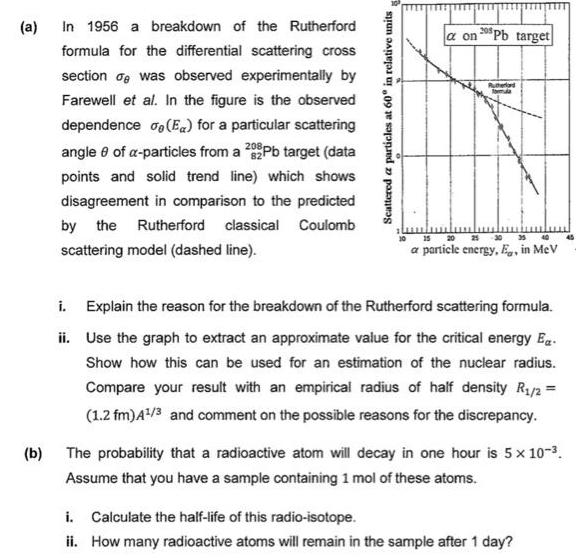
(a) (b) In 1956 a breakdown of the Rutherford formula for the differential scattering cross section was observed experimentally by Farewell et al. In the figure is the observed dependence (Ex) for a particular scattering angle 8 of a-particles from a 289Pb target (data points and solid trend line) which shows disagreement in comparison to the predicted by the Rutherford classical Coulomb scattering model (dashed line). Scattered a particles at 60 in relative units a on 20 Pb target 20 25 30 35 40 a particle energy, Eg, in MeV ii. i. Explain the reason for the breakdown of the Rutherford scattering formula. Use the graph to extract an approximate value for the critical energy E. Show how this can be used for an estimation of the nuclear radius. Compare your result with an empirical radius of half density R/2 = (1.2 fm) 4/3 and comment on the possible reasons for the discrepancy. The probability that a radioactive atom will decay in one hour is 5 x 10-. Assume that you have a sample containing 1 mol of these atoms. i. Calculate the half-life of this radio-isotope. ii. How many radioactive atoms will remain in the sample after 1 day?
Step by Step Solution
★★★★★
3.52 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


