Answered step by step
Verified Expert Solution
Question
1 Approved Answer
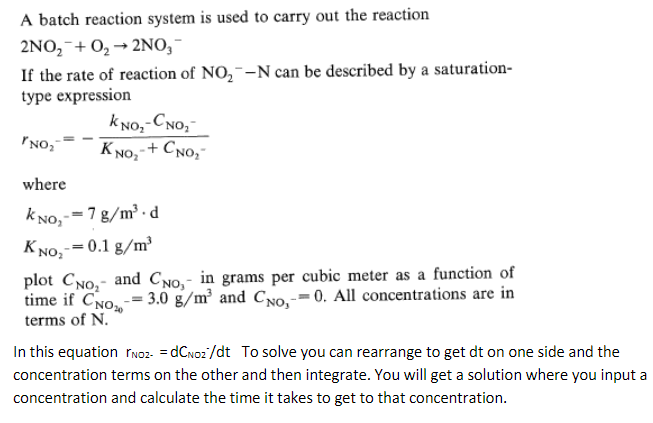
A batch reaction system is used to carry out the reaction 2 N O 2 - + O 2 2 N O 3 - If
A batch reaction system is used to carry out the reaction
If the rate of reaction of can be described by a saturation
type expression
where
plot and in grams per cubic meter as a function of
time if and All concentrations are in
terms of
In this equation To solve you can rearrange to get on one side and the
concentration terms on the other and then integrate. You will get a solution where you input a
concentration and calculate the time it takes to get to that concentration.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


