Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A bomb calorimeter is filled with 8.2 atm of an unknown gaseous hydrocarbon fuel and an excess of oxygen gas at a temperature of 20.8
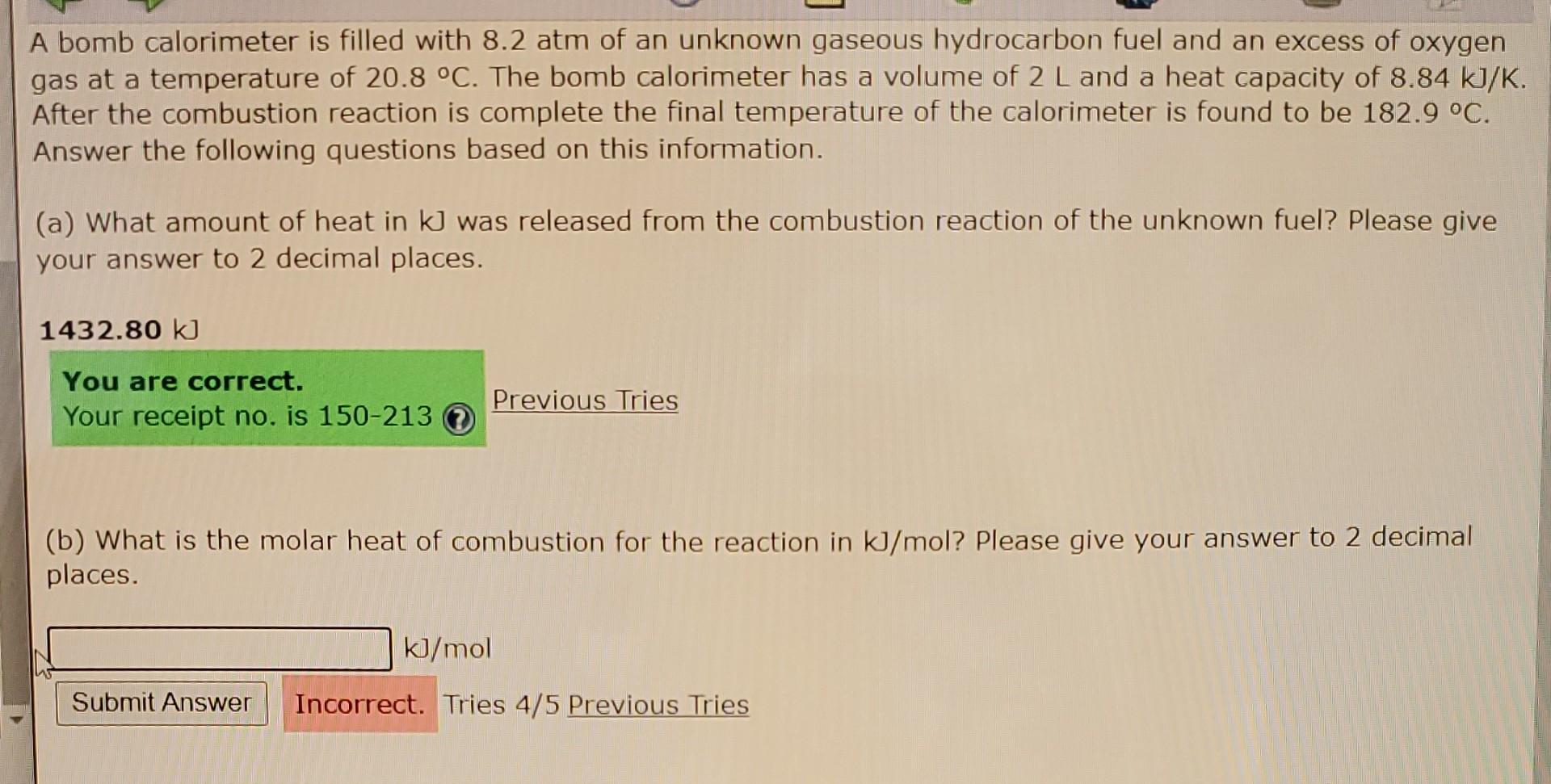
A bomb calorimeter is filled with 8.2 atm of an unknown gaseous hydrocarbon fuel and an excess of oxygen gas at a temperature of 20.8 C. The bomb calorimeter has a volume of 2 L and a heat capacity of 8.84 kJ/K. After the combustion reaction is complete the final temperature of the calorimeter is found to be 182.9 C. Answer the following questions based on this information. (a) What amount of heat in kJ was released from the combustion reaction of the unknown fuel? Please give your answer to 2 decimal places. 1432.80 kJ You are correct. Your receipt no. is 150-213 Previous Tries (b) What is the molar heat of combustion for the reaction in kJ/mol? Please give your answer to 2 decimal places. kJ/mol Submit Answer Incorrect. Tries 4/5 Previous Tries
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


