Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) Calculate the concentration of initiator required to prepare polystyrene with a number average molar mass of 750kg/mol by bulk polymerization at 60C. Assume that
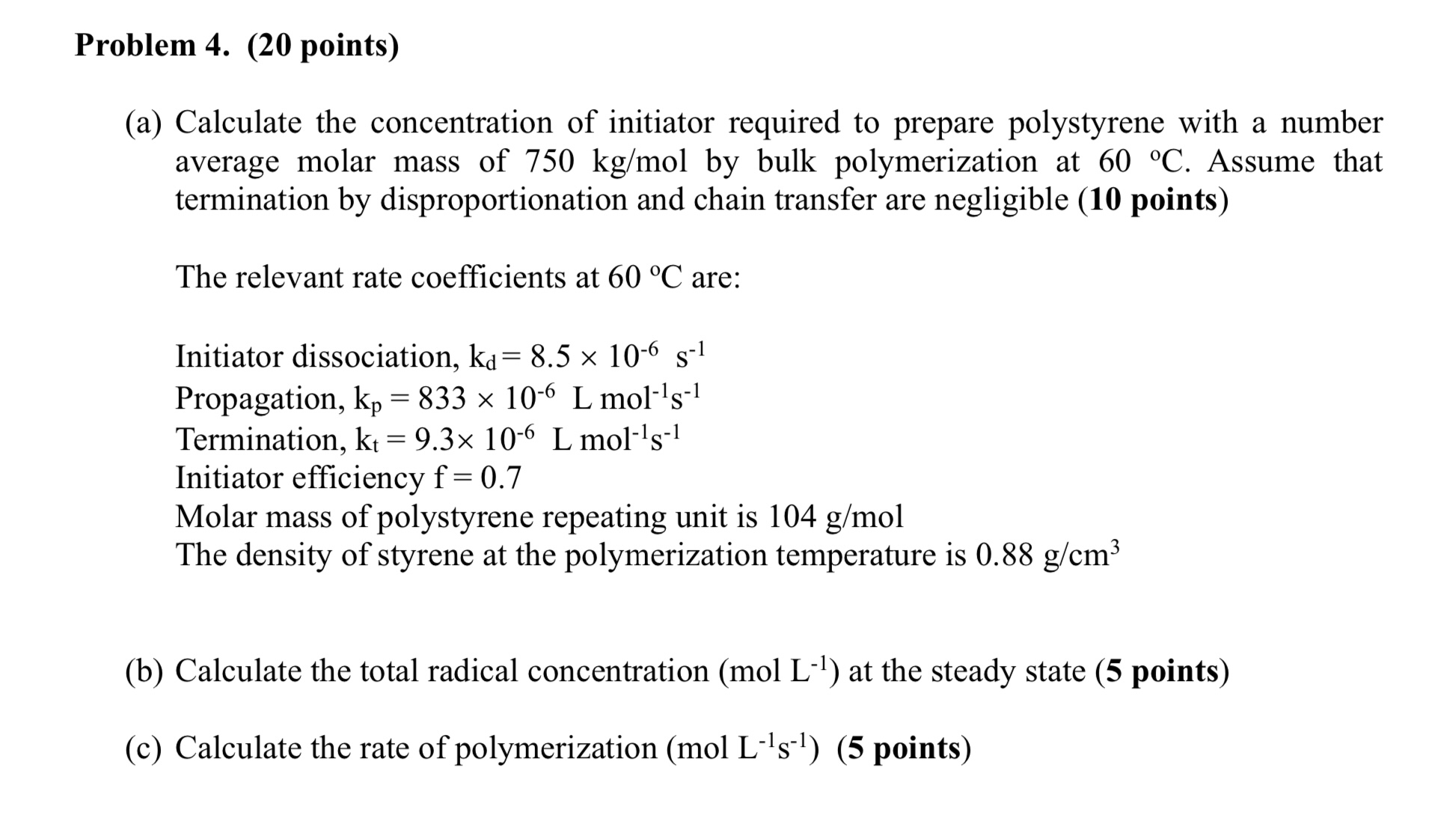 (a) Calculate the concentration of initiator required to prepare polystyrene with a number average molar mass of 750kg/mol by bulk polymerization at 60C. Assume that termination by disproportionation and chain transfer are negligible (10 points) The relevant rate coefficients at 60C are: Initiator dissociation, kd=8.5106s1 Propagation, kp=833106Lmol1s1 Termination, kt=9.3106Lmol1s1 Initiator efficiency f=0.7 Molar mass of polystyrene repeating unit is 104g/mol The density of styrene at the polymerization temperature is 0.88g/cm3 (b) Calculate the total radical concentration (molL1) at the steady state (5 points) (c) Calculate the rate of polymerization (molL1s1) (5 points)
(a) Calculate the concentration of initiator required to prepare polystyrene with a number average molar mass of 750kg/mol by bulk polymerization at 60C. Assume that termination by disproportionation and chain transfer are negligible (10 points) The relevant rate coefficients at 60C are: Initiator dissociation, kd=8.5106s1 Propagation, kp=833106Lmol1s1 Termination, kt=9.3106Lmol1s1 Initiator efficiency f=0.7 Molar mass of polystyrene repeating unit is 104g/mol The density of styrene at the polymerization temperature is 0.88g/cm3 (b) Calculate the total radical concentration (molL1) at the steady state (5 points) (c) Calculate the rate of polymerization (molL1s1) (5 points) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


