Question
2. Based on the following balanced equation, 2 Fe(s) + 3 CuSO4(aq) a. What is the charge on the iron in the product, Fe(SO4)?
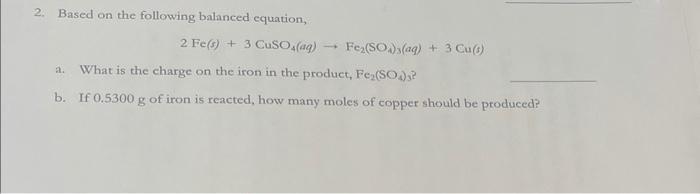
2. Based on the following balanced equation, 2 Fe(s) + 3 CuSO4(aq) a. What is the charge on the iron in the product, Fe(SO4)? b. If 0.5300 g of iron is reacted, how many moles of copper should be produced? 1 Fe(SO)(aq) + + 3 Cu(6)
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
Consider the reaction 2 fe s Let charge on se x S 6 02 s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cost Management Measuring Monitoring And Motivating Performance
Authors: Leslie G. Eldenburg, Susan K. Wolcott
2nd Edition
978-0-470-7694, 0470769424, 978-0470769423
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



