Question
A. Calculate the nuclear binding energy per nucleon of the following isotopes: Isotopes He (4.0026 amu) 194W (183.9510 amu) Number 1 2 B. Calculate
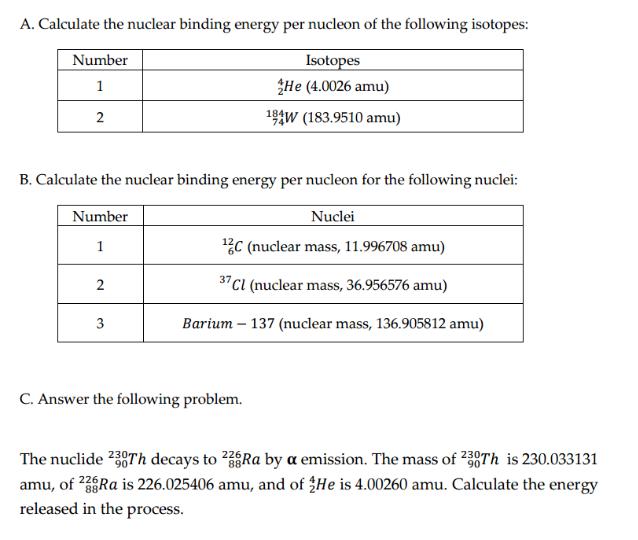
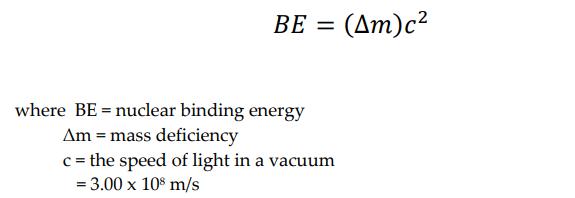
A. Calculate the nuclear binding energy per nucleon of the following isotopes: Isotopes He (4.0026 amu) 194W (183.9510 amu) Number 1 2 B. Calculate the nuclear binding energy per nucleon for the following nuclei: Number Nuclei 1 2C (nuclear mass, 11.996708 amu) 37 Cl (nuclear mass, 36.956576 amu) Barium - 137 (nuclear mass, 136.905812 amu) 2 3 C. Answer the following problem. 226 The nuclide 230Th decays to 238Ra by a emission. The mass of 230Th is 230.033131 amu, of 23Ra is 226.025406 amu, and of He is 4.00260 amu. Calculate the energy released in the process. BE = (m)c2 where BE = nuclear binding energy Am = mass deficiency c = the speed of light in a vacuum = 3.00 x 108 m/s
Step by Step Solution
3.53 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



