Answered step by step
Verified Expert Solution
Question
1 Approved Answer
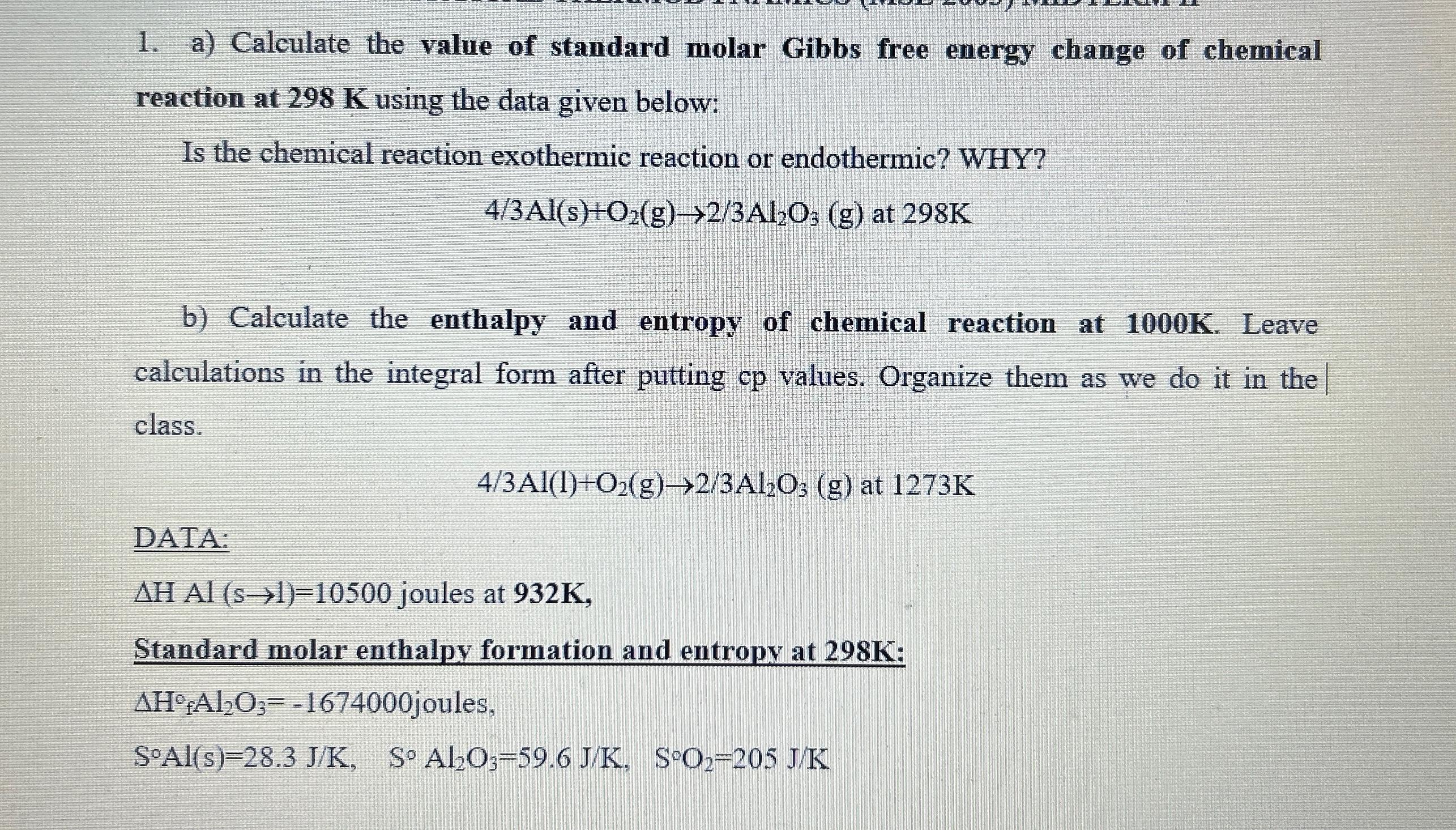
a ) Calculate the value of standard molar Gibbs free energy change of chemical reaction at 2 9 8 K using the data given below:
a Calculate the value of standard molar Gibbs free energy change of chemical reaction at using the data given below:
Is the chemical reaction exothermic reaction or endothermic? WHY?
b Calculate the enthalpy and entropy of chemical reaction at Leave calculations in the integral form after putting values. Organize them as we do it in the class.
DATA:
joules at
Standard molar enthalpy formation and entropy at :
joules,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


