Question
A catalytic reaction 2A(g)->B(g) is investegated in a fluid bed reactor. The reactor is isothermic, and the reaction mixture is ideal mixed. Therefore the concentration
A catalytic reaction 2A(g)->B(g) is investegated in a fluid bed reactor. The reactor is isothermic, and the reaction mixture is ideal mixed. Therefore the concentration is the same, through the reactor. The reactor is a CSTR (mixed flow reactor)
The feed mixture is going in to the reactor continuing, so when steady state happens the conversion X can be mesured at the exit of the reactor.
the reactor is used to investigate the reaction kinetics, knowing constant temperature and pressure can messure cohesive values from the volumetric feed rate v0, and the conversion X. the catalyst is powdered, so there wont be any gas film resistance, or pore diffusion resistance.
Following data is given:
Feed rate: pure A gas. ideal gas law applies
Temperature: 300 degrees celcius
pressure: 1,013*105 Pa
catalyst mass: 0,2 kg
and the following table:
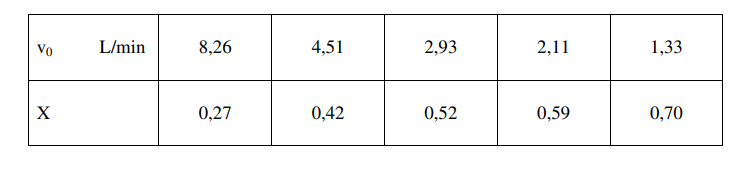
1) make a stochiometric table by using molar flows, and express the concentration of A and B by the initial concentration A(CA0) and the conversion og A (X)
2) calculate cohesive values of the concentration of A (CA) and the reaction rate whereby A is transformed (-rA)
3) show that there is a linear connection between ln(-rA) og ln(CA). then use this connection to determinate the reaction order for the follow expression:
-rA=kCAn
and calculate the rate constant (k) with units
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


