Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemical equilibrium is established when methane gas reacts with hydrogen sulfide gas in a round glass flask to produce solid carbon disulfide and
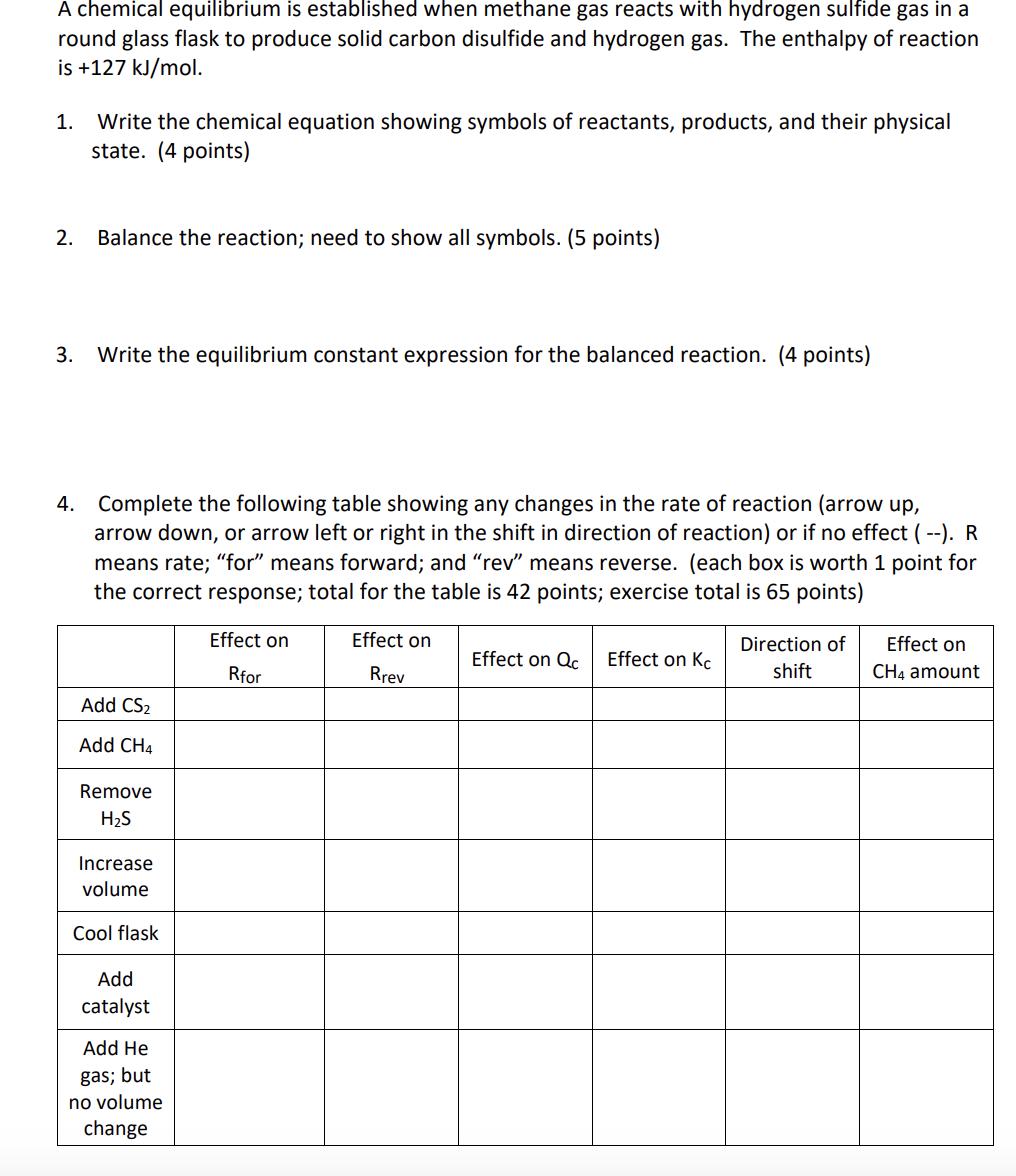
A chemical equilibrium is established when methane gas reacts with hydrogen sulfide gas in a round glass flask to produce solid carbon disulfide and hydrogen gas. The enthalpy of reaction is +127 kJ/mol. 1. Write the chemical equation showing symbols of reactants, products, and their physical state. (4 points) 2. Balance the reaction; need to show all symbols. (5 points) 3. Write the equilibrium constant expression for the balanced reaction. (4 points) 4. Complete the following table showing any changes in the rate of reaction (arrow up, arrow down, or arrow left or right in the shift in direction of reaction) or if no effect (--). R means rate; "for" means forward; and "rev" means reverse. (each box is worth 1 point for the correct response; total for the table is 42 points; exercise total is 65 points) Effect on Rfor Add CS2 Add CH4 Remove HS Increase volume Cool flask Add catalyst Add He gas; but no volume change Effect on Rrev Effect on Qc Effect on Kc Direction of shift Effect on CH4 amount
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


