Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemical plant produces waste gas that is used as a fuel for heating. The volumetric composition of the fuel is: Methane (CH4) =
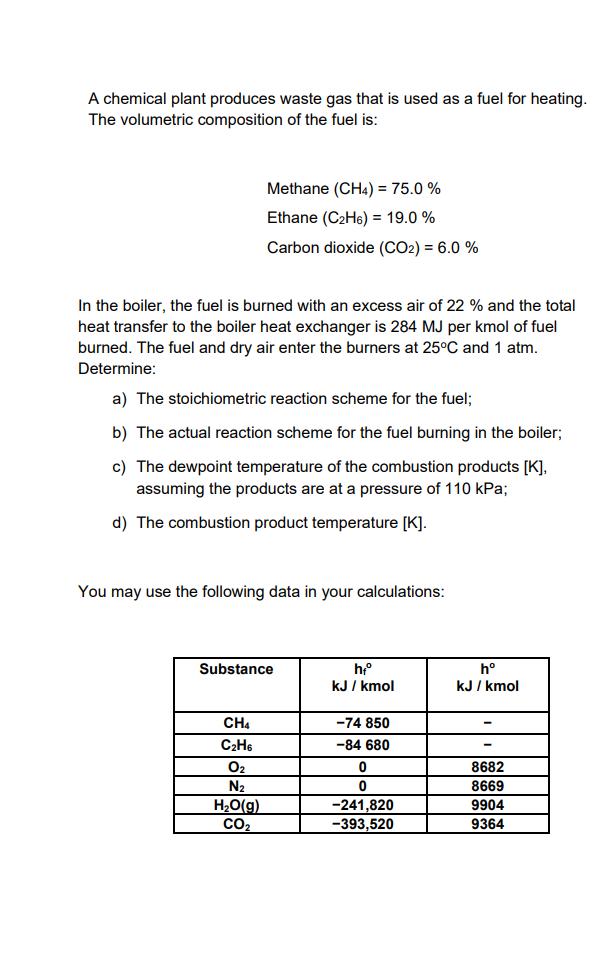
A chemical plant produces waste gas that is used as a fuel for heating. The volumetric composition of the fuel is: Methane (CH4) = 75.0 % Ethane (CH6) = 19.0 % Carbon dioxide (CO2) = 6.0 % In the boiler, the fuel is burned with an excess air of 22 % and the total heat transfer to the boiler heat exchanger is 284 MJ per kmol of fuel burned. The fuel and dry air enter the burners at 25C and 1 atm. Determine: a) The stoichiometric reaction scheme for the fuel; b) The actual reaction scheme for the fuel burning in the boiler; c) The dewpoint temperature of the combustion products [K], assuming the products are at a pressure of 110 kPa; d) The combustion product temperature [K]. You may use the following data in your calculations: Substance CH4 CH6 0 N HO(g) CO hf kJ/kmol -74 850 -84 680 0 0 -241,820 -393,520 h kJ/kmol 8682 8669 9904 9364
Step by Step Solution
★★★★★
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
a The stoichiometric reaction scheme for the fuel is CH4 2O2 CO2 2H...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


