Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemist designs a galvanic cell that uses these two half-reactions: half-reaction N(9)+4HO(1)+4e Answer the following questions about this cell. Write a balanced equation
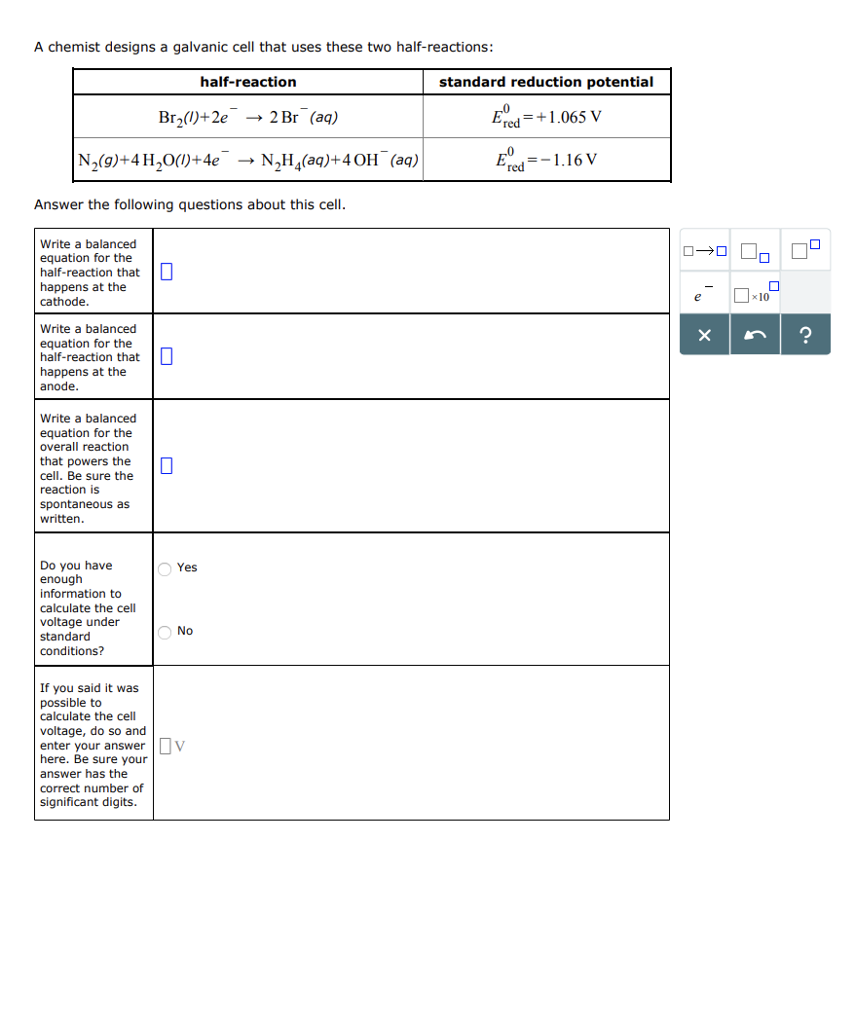
A chemist designs a galvanic cell that uses these two half-reactions: half-reaction N(9)+4HO(1)+4e Answer the following questions about this cell. Write a balanced equation for the half-reaction that 0 happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is Br(1)+2e 2 Br (aq) spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? If you said it was possible to calculate the cell voltage, do so and 0 Yes O No NH(aq)+4 OH(aq) enter your answer v here. Be sure your answer has the correct number of significant digits. standard reduction potential =+1.065 V Ered Ered =-1.16 V 0-000 X 0 x10 ?
Step by Step Solution
★★★★★
3.46 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Br 1 2e 2Br 1 U 4Br ag Exed 1 065V 2Br as Cathode reduction b N ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


