Answered step by step
Verified Expert Solution
Question
1 Approved Answer
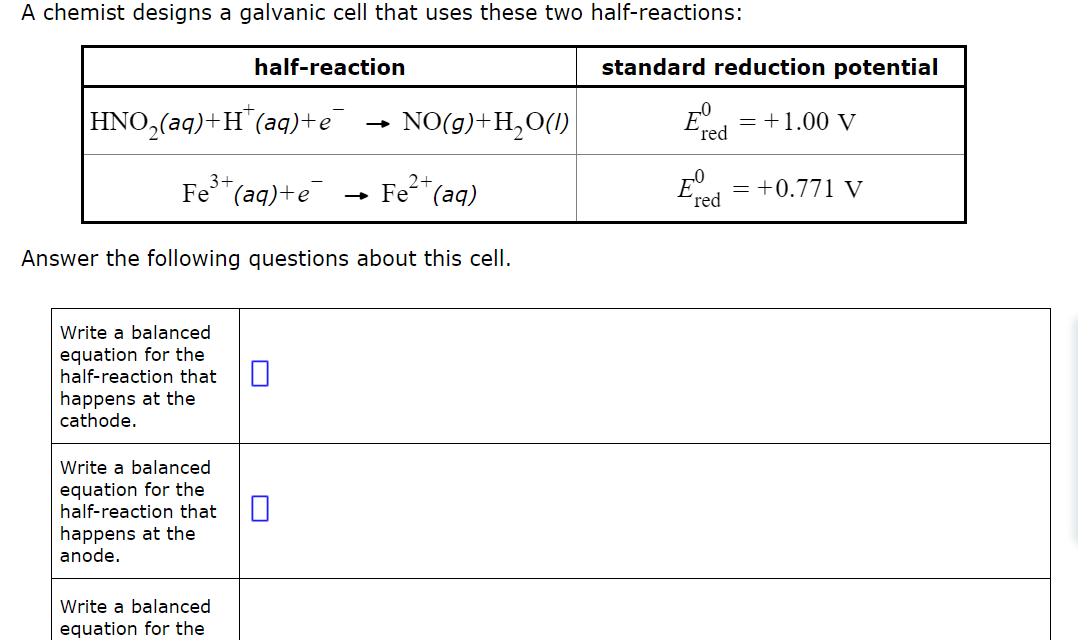
A chemist designs a galvanic cell that uses these two half - reactions: Write a balanced equation for the half - reaction that happens at
A chemist designs a galvanic cell that uses these two halfreactions:
Write a balanced equation for the halfreaction that happens at the cathode.Write a balanced equation for the halfreaction that happens at the anode.Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written.If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


