Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH, (g) =C,H,(g) + 3 H,
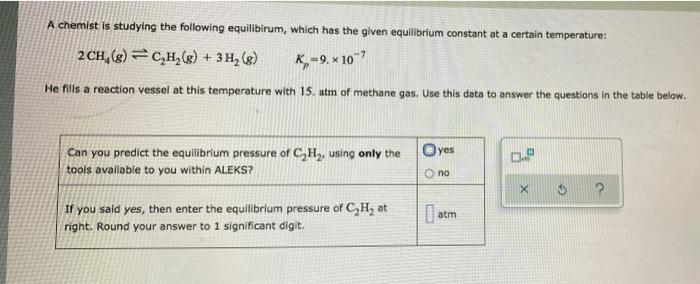
A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH, (g) =C,H,(g) + 3 H, (g) K=9, x 10"7 He fills a reaction vessel at this temperature with 15. atm of methane gas. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of C,H2, using only the Oyes tools available to you within ALEKS? O no If you sald yes, then enter the equilibrium pressure of C,H, at | atm right. Round your answer to 1 significant digit.
Step by Step Solution
★★★★★
3.46 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636840bc52cfb_241854.pdf
180 KBs PDF File
636840bc52cfb_241854.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


