Question
A chromatogram of a two-component mixture on a 25-cm packed LC column is shown in the figure below. The flow rate was 0.40 ml/min. (a)
A chromatogram of a two-component mixture on a 25-cm packed LC column is shown in the figure below. The flow rate was 0.40 ml/min.
(a) Find the times that components A and B spend in the stationary phase
.(b) Find the retention times for A and B.
(c) Determine the retention factors for the two components.
(d) Find the full widths of each peak and the full width at half-maximum values.
(e) Find the resolution of the two peaks.
f) Find the average number of plates for the column.
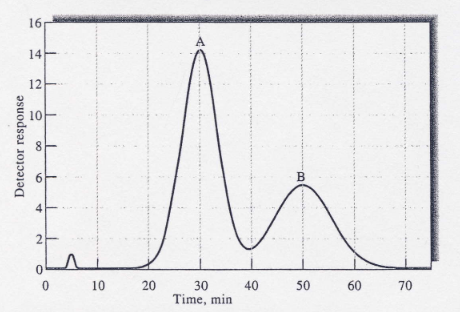
(g) Find the average plate height.
(h) What column length would be needed to achieve a resolution of 1.75?
(i) What time would be required to achieve the resolution in part (h)?
( j) Assume that the column length is fixed at 25 cm and the packing material is fixed.
What measures could you take to increase the resolution to achieve baseline separation?
(k) Are there any measures you could use to achieve a better separation in a shorter time with the same column as in part (j)?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


