Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A clear handwriting without an explanation please. 2. Nitromethane (1) and acetonitrile (2) form an ideal solution at low pressures. Their vapor pressures have been
A clear handwriting without an explanation please. 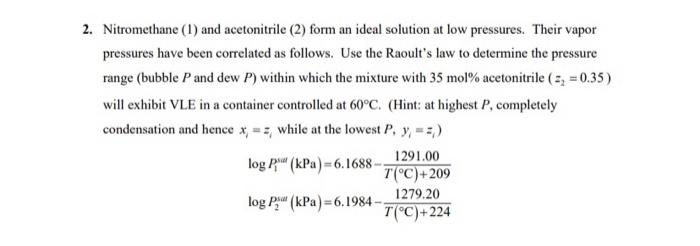
2. Nitromethane (1) and acetonitrile (2) form an ideal solution at low pressures. Their vapor pressures have been correlated as follows. Use the Raoult's law to determine the pressure range (bubble P and dew P) within which the mixture with 35 mol% acetonitrile ( 52 = 0.35) will exhibit VLE in a container controlled at 60C. (Hint: at highest P, completely condensation and hence x = =, while at the lowest P. y - :) log Poul (kPa) -6.1688 - 1291.00 T(C)+209 1279.20 log P (kPa)=6.1984- T(C)+224 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


