Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A closed system consists of 0.3kmol of octane occupying a volume of 2m^(3) . Determine (a) the weight of the system, in N , and
A closed system consists of
0.3kmolof octane occupying a volume of
2m^(3). Determine (a) the weight of the system, in
N, and (b) the molar-based and mass-based specific volumes, in
(m^(3))/(k)moland
(m^(3))/(k)grespectively. Let
g=9.81(m)/(s^(2)).\ Your answer is incorrect.\ Determine the weight of the system, in
N.\
F_(grav )=|_(_())N|\ Hint\ Your answer is partially correct.\ Determine the molar-based and mass-based specific volumes, in
(m^(3))/(k)moland
(m^(3))/(k)grespectively.\
/bar (v)=\ (m^(3))/(k)mol\ v=\ (m^(3))/(k)g 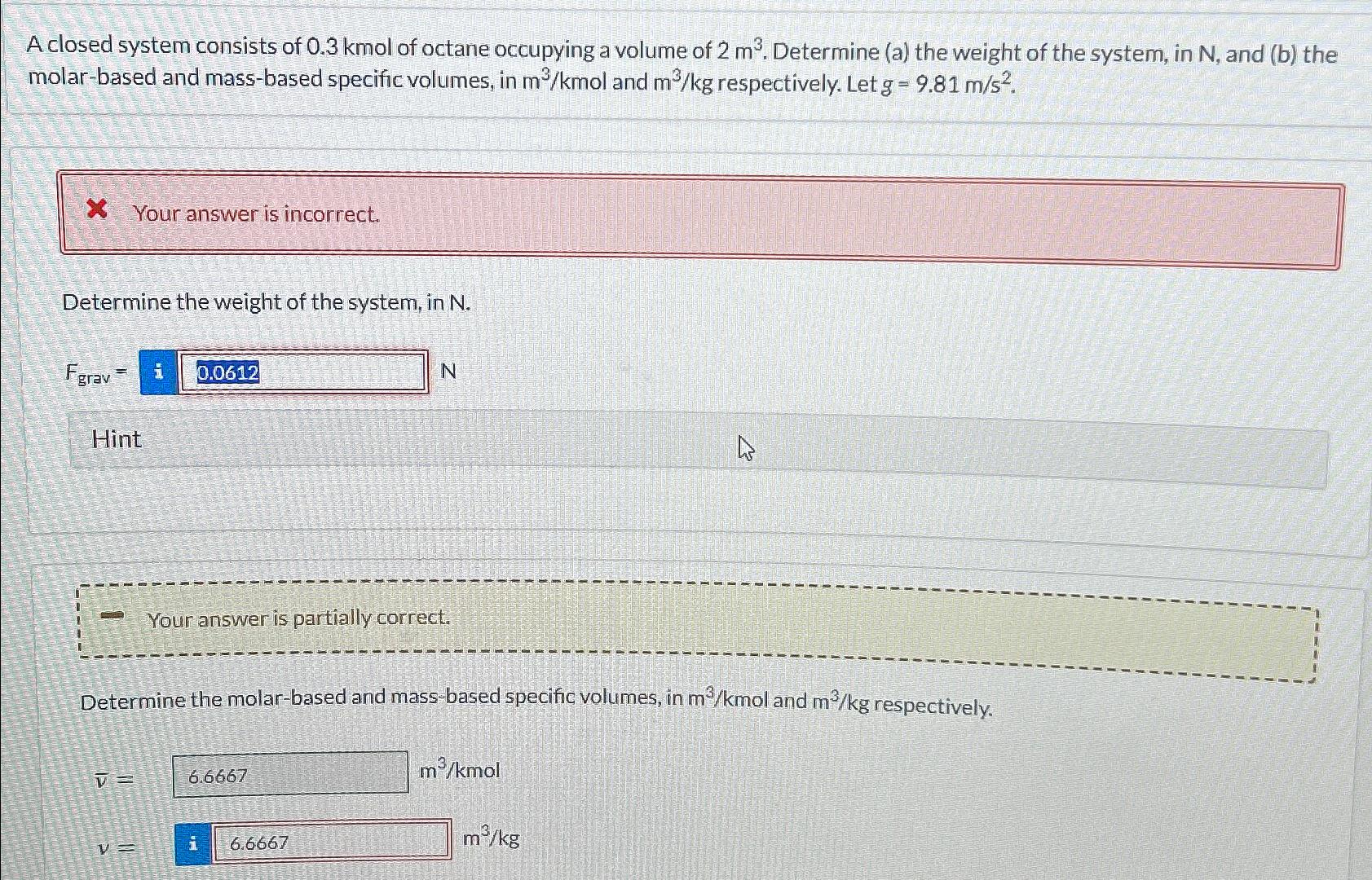
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


