Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A coal sample from Sarawak has an ultimate analysis by mass as shown in TABLE Q4. The coal is burned with 40% access air.
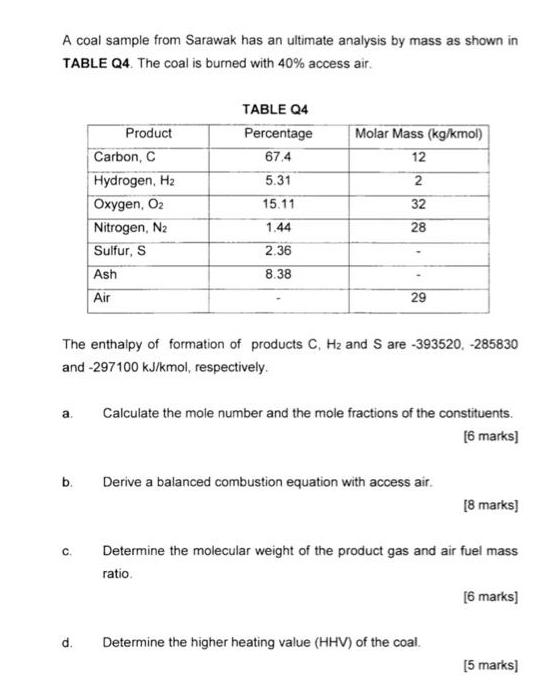
A coal sample from Sarawak has an ultimate analysis by mass as shown in TABLE Q4. The coal is burned with 40% access air. a. b. C. Product d. Carbon, C Hydrogen, H Oxygen, O Nitrogen, N Sulfur, S Ash Air The enthalpy of formation of products C, H and S are -393520, -285830 and -297100 kJ/kmol, respectively. TABLE Q4 Percentage 67.4 5.31 15.11 1.44 2.36 8.38 Molar Mass (kg/kmol) 12 2 32 28 29 Calculate the mole number and the mole fractions of the constituents. [6 marks] Derive a balanced combustion equation with access air. [8 marks] Determine the molecular weight of the product gas and air fuel mass ratio. Determine the higher heating value (HHV) of the coal. [6 marks] [5 marks]
Step by Step Solution
★★★★★
3.32 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
a To calculate the mole number and mole fractions of the constituents we need to convert the given percentages to mole fractions First lets calculate the mass of each constituent based on the given pe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


