Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A combustion gas mixture of oxygen and hydrogen at 3466 K and 10.34 MPa (1500 psi) has the composition shown in the table below.
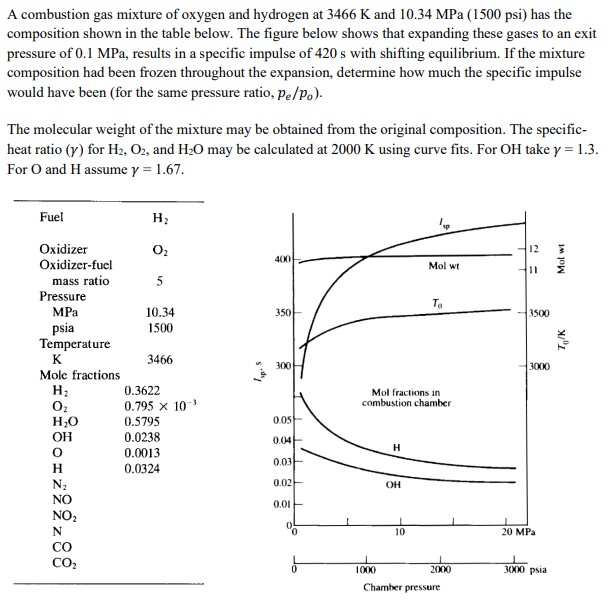
A combustion gas mixture of oxygen and hydrogen at 3466 K and 10.34 MPa (1500 psi) has the composition shown in the table below. The figure below shows that expanding these gases to an exit pressure of 0.1 MPa, results in a specific impulse of 420 s with shifting equilibrium. If the mixture composition had been frozen throughout the expansion, determine how much the specific impulse would have been (for the same pressure ratio, pe/Po). The molecular weight of the mixture may be obtained from the original composition. The specific- heat ratio (y) for H2, O2, and H2O may be calculated at 2000 K using curve fits. For OH take y = 1.3. For O and H assume y = 1.67. Mol wt Fuel Oxidizer H 02 12 400 Oxidizer-fuel Mol wt 11 mass ratio 5 Pressure To MPa 10.34 350 3500 psia 1500 Temperature K 3466 300 3000 Mole fractions H 0.3622 Oz 0.795 x 10-3 Mol fractions in combustion chamber HO 0.5795 0.05 OH 0.0238 0.04- H 0.0013 0.03 H 0.0324 N 0.02 OH NO 0.01 NO2 N CO CO 1000 10 Chamber pressure 20 MPa 2000 3000 psia To/K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


