Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A combustion reactor for methane (CH4) is fed with 10mol%CH4,20mol%O2 and 70 mol%N2. The reactions occurring in the combustion reactor are: CH4+3/2O2CO+2H2OCH4+2O2CO2+2H2O It is given
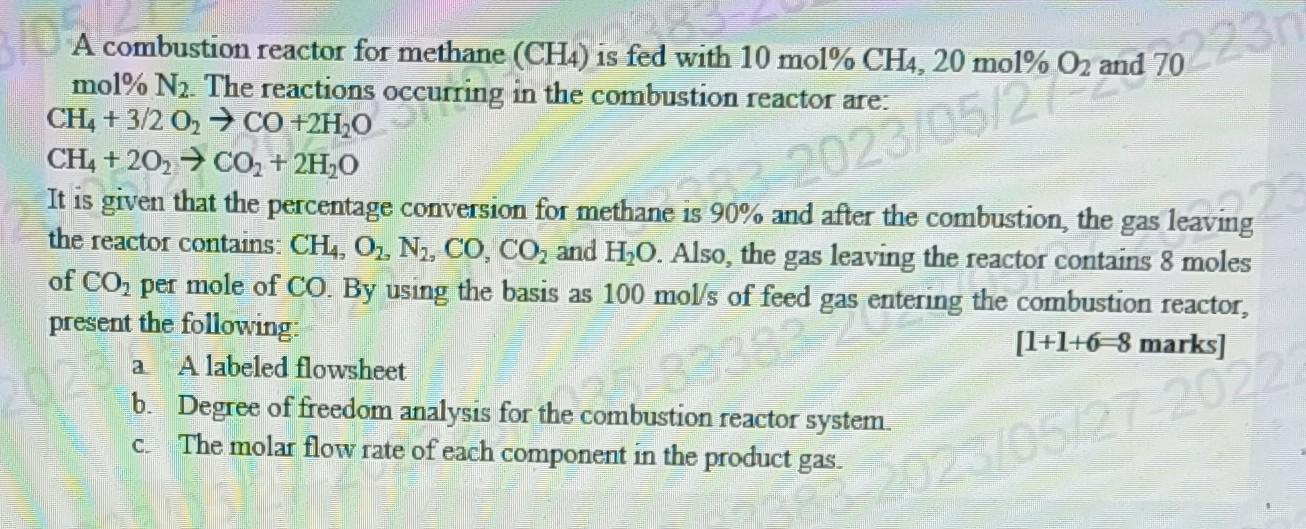
A combustion reactor for methane (CH4) is fed with 10mol%CH4,20mol%O2 and 70 mol%N2. The reactions occurring in the combustion reactor are: CH4+3/2O2CO+2H2OCH4+2O2CO2+2H2O It is given that the percentage conversion for methane is 90% and after the combustion, the gas leaving the reactor contains: CH4,O2,N2,CO,CO2 and H2O. Also, the gas leaving the reactor contains 8 moles of CO2 per mole of CO. By using the basis as 100mol/s of feed gas entering the combustion reactor, present the following: a. A labeled flowsheet [1+1+6=8marks] b. Degree of freedom analysis for the combustion reactor system c. The molar flow rate of each component in the product gas
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


