Question
A compound is analyzed and found to be 64.80% C, 13.62% H, and 21,58% O. What is the chemical formula of this compound? Enter
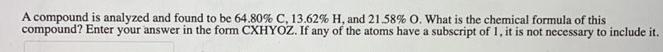
A compound is analyzed and found to be 64.80% C, 13.62% H, and 21,58% O. What is the chemical formula of this compound? Enter your answer in the form CXHYOZ. If any of the atoms have a subscript of 1, it is not necessary to include it.
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Solution Basis 100 g of compound Compounds are assume as 6480 C 1362 H 2158 O Carbon 648 g moles of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



