Question
A compressed air tank in a mechanic shop holds a volume of 1 m of air at 1000 kPa. The temperature of the air
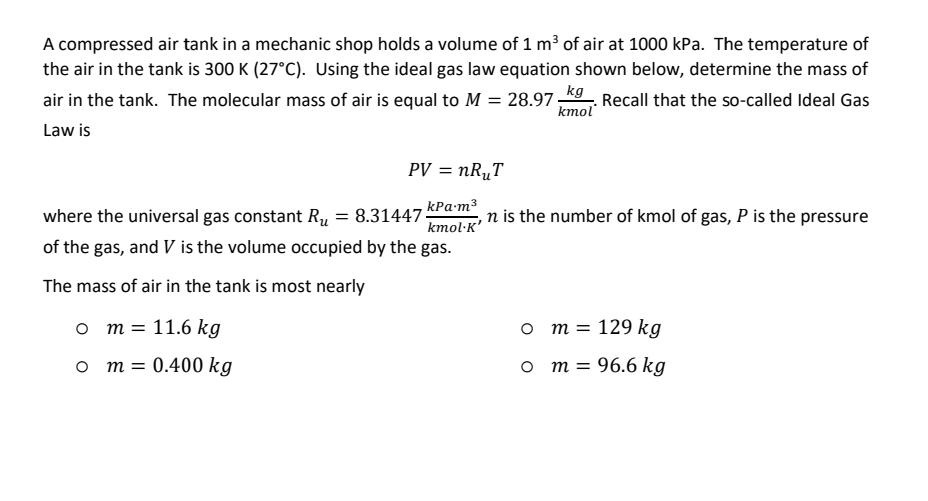
A compressed air tank in a mechanic shop holds a volume of 1 m of air at 1000 kPa. The temperature of the air in the tank is 300 K (27C). Using the ideal gas law equation shown below, determine the mass of air in the tank. The molecular mass of air is equal to M = 28.97 kg. Recall that the so-called Ideal Gas Law is kmol PV = nRT kPa.m kmolK' where the universal gas constant R = 8.31447- of the gas, and V is the volume occupied by the gas. The mass of air in the tank is most nearly O m = 11.6 kg O m = 0.400 kg n is the number of kmol of gas, P is the pressure O m = 129 kg m = 96.6 kg
Step by Step Solution
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below The problem statemen...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



