Question
(a) Compute the particle-phase fraction of a component with volatility = 2 3 under organic aerosol mass loading, M = 0.2, 2, 20, 200 3.
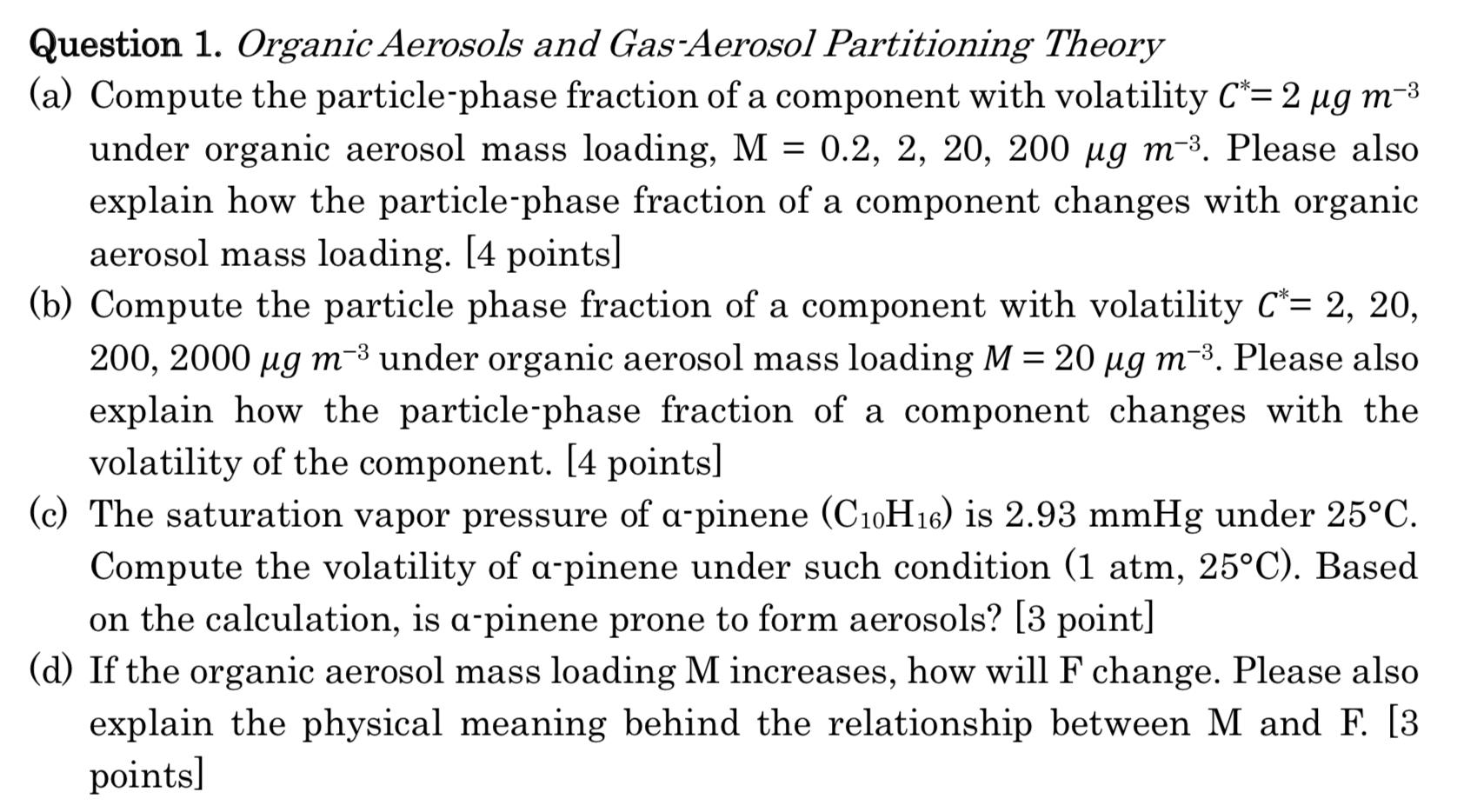 (a) Compute the particle-phase fraction of a component with volatility = 2 3 under organic aerosol mass loading, M = 0.2, 2, 20, 200 3. Please also explain how the particle-phase fraction of a component changes with organic aerosol mass loading. [4 points] (b) Compute the particle phase fraction of a component with volatility = 2, 20, 200, 2000 3 under organic aerosol mass loading = 20 3. Please also explain how the particle-phase fraction of a component changes with the volatility of the component. [4 points] (c) The saturation vapor pressure of -pinene (C10H16) is 2.93 mmHg under 25C. Compute the volatility of -pinene under such condition (1 atm, 25C). Based on the calculation, is -pinene prone to form aerosols? [3 point] (d) If the organic aerosol mass loading M increases, how will F change. Please also explain the physical meaning behind the relationship between M and F. [3 points]
(a) Compute the particle-phase fraction of a component with volatility = 2 3 under organic aerosol mass loading, M = 0.2, 2, 20, 200 3. Please also explain how the particle-phase fraction of a component changes with organic aerosol mass loading. [4 points] (b) Compute the particle phase fraction of a component with volatility = 2, 20, 200, 2000 3 under organic aerosol mass loading = 20 3. Please also explain how the particle-phase fraction of a component changes with the volatility of the component. [4 points] (c) The saturation vapor pressure of -pinene (C10H16) is 2.93 mmHg under 25C. Compute the volatility of -pinene under such condition (1 atm, 25C). Based on the calculation, is -pinene prone to form aerosols? [3 point] (d) If the organic aerosol mass loading M increases, how will F change. Please also explain the physical meaning behind the relationship between M and F. [3 points]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


