Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. Concentration of Reagents B. Reaction Times table[[Mixture,Trial 1,Trial 2,table[[Average Time],[(seconds)]]],[1, 28.92Sec- ,,],[2, 36.82Sec ,,],[3, 49.42Sec ,,],[4, 1min18Sec ,,],[5, 2min29Sec ,,]] II. Calculations Show
A. Concentration of Reagents\ \ B. Reaction Times\ \\\\table[[Mixture,Trial 1,Trial 2,\\\\table[[Average Time],[(seconds)]]],[1,
28.92Sec-,,],[2,
36.82Sec,,],[3,
49.42Sec,,],[4,
1min18Sec,,],[5,
2min29Sec,,]]\ II. Calculations\ Show a sample calculation for reaction mixture 3. Show any equations and units.\ A. Calculation of
IO_(3)^(-)in mixture 3 .\ III. Tabulation of Data for Graph\ A. Graph to Determine Reaction Order\ \\\\table[[Mixture, time),
IO_(3)^(-),
log[IO_(3)^(-)] 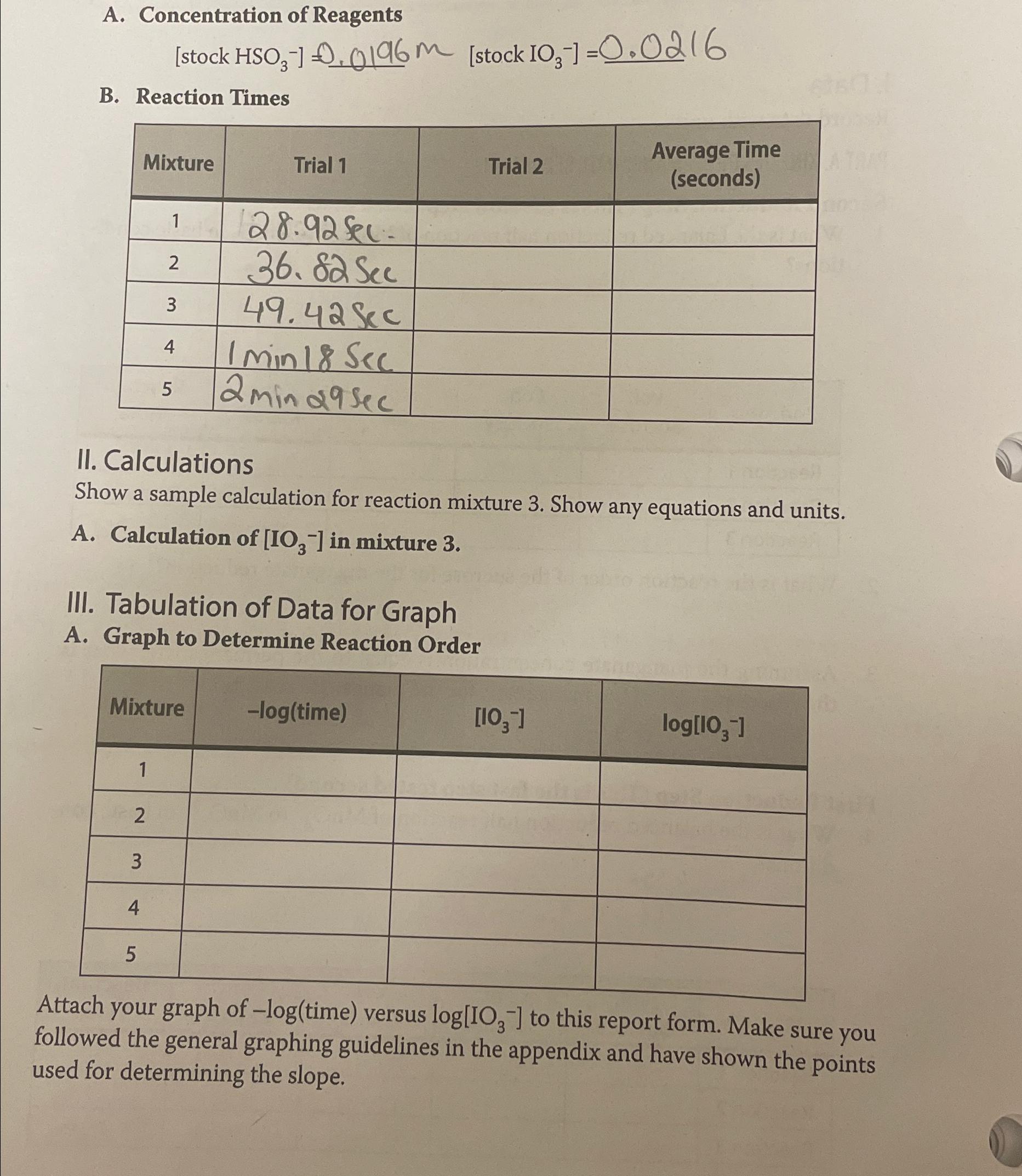
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


