Answered step by step
Verified Expert Solution
Question
1 Approved Answer
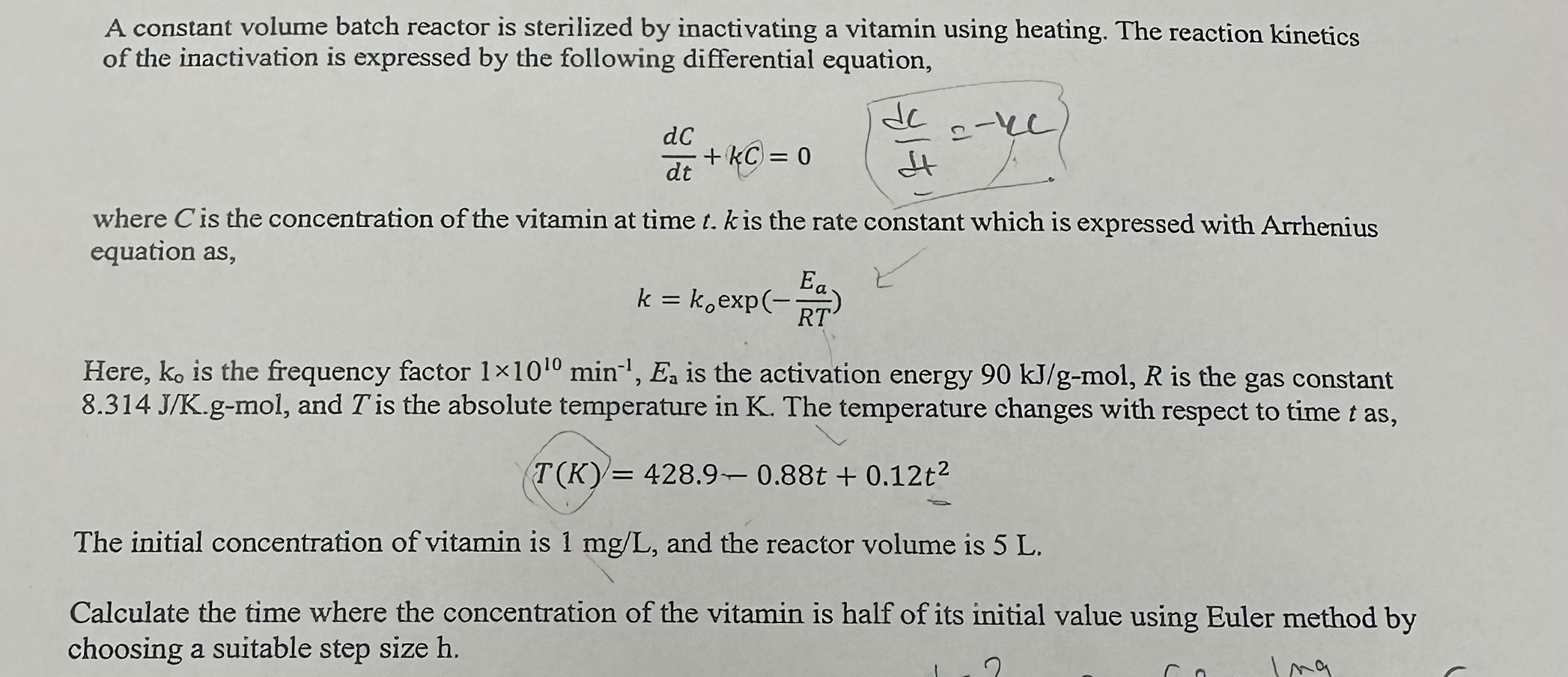
A constant volume batch reactor is sterilized by inactivating a vitamin using heating. The reaction kinetics of the inactivation is expressed by the following differential
A constant volume batch reactor is sterilized by inactivating a vitamin using heating. The reaction kinetics of the inactivation is expressed by the following differential equation,
where is the concentration of the vitamin at time is the rate constant which is expressed with Arrhenius equation as
exp
Here, is the frequency factor is the activation energy mol, is the gas constant gmol, and is the absolute temperature in The temperature changes with respect to time as
The initial concentration of vitamin is and the reactor volume is
Calculate the time where the concentration of the vitamin is half of its initial value using Euler method by choosing a suitable step size hDO NOT CALCULATE BY USING CODE, calculate it analytically.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


