Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A container with 5.5 mol of oxygen gas at 300 K is placed on a large stone slab with a temperature of 600 K.
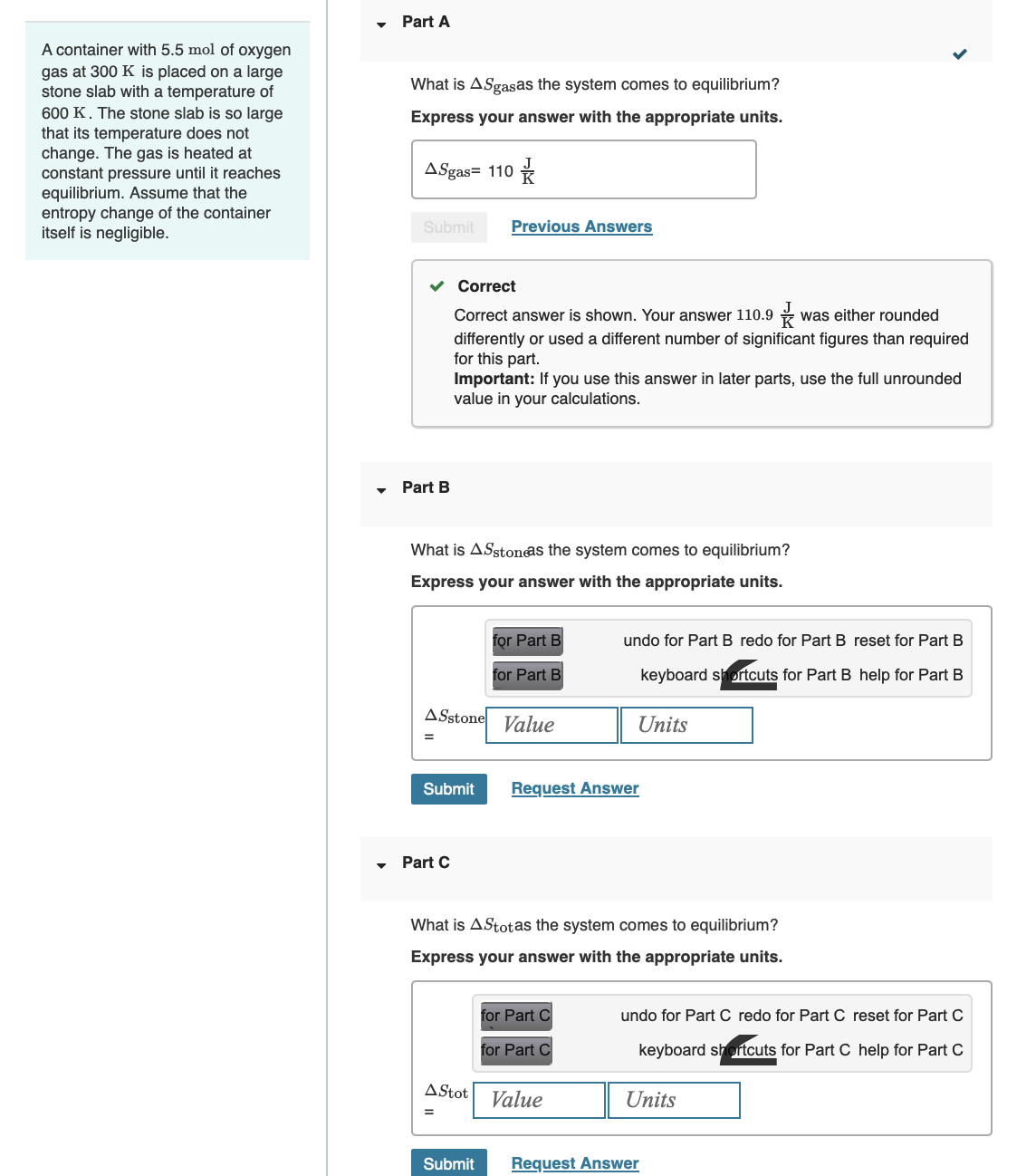
A container with 5.5 mol of oxygen gas at 300 K is placed on a large stone slab with a temperature of 600 K. The stone slab is so large that its temperature does not change. The gas is heated at constant pressure until it reaches equilibrium. Assume that the entropy change of the container itself is negligible. Part A What is ASgasas the system comes to equilibrium? Express your answer with the appropriate units. ASgas= 110 Submit Previous Answers Correct Part B Correct answer is shown. Your answer 110.9 was either rounded differently or used a different number of significant figures than required for this part. Important: If you use this answer in later parts, use the full unrounded value in your calculations. What is AS stone as the system comes to equilibrium? Express your answer with the appropriate units. for Part B for Part B undo for Part B redo for Part B reset for Part B keyboard shortcuts for Part B help for Part B ASstone Value Units Submit Request Answer Part C What is Astotas the system comes to equilibrium? Express your answer with the appropriate units. for Part C for Part C A Stot Value undo for Part C redo for Part C reset for Part C keyboard shortcuts for Part C help for Part C Units Submit Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Part B To calculate the change in entropy of the stone slab ASstone as the system come...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


