Question
A corrosion test was performed on a pure metal M in de-aerated pH 2 sulphuric acid. The rates of both oxidation and reduction half reactions
A corrosion test was performed on a pure metal M in de-aerated pH 2 sulphuric acid. The rates of both oxidation and reduction half reactions are controlled by activation polarization and the data are tabulated in below table. Assume activation control and concentration of M2+ = 10-6 mole/litre.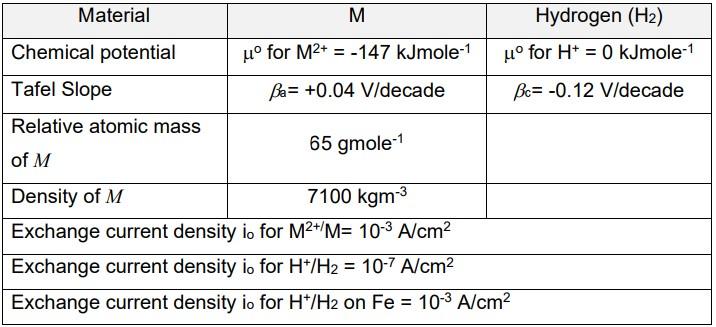
Table A : Data for anodic and cathodic reactant
a) Evaluate the corrosion rate of a pure metal M in de-aerated pH 2 sulphuric acid.
b) Assess the possible consequences of corrosion current density if the system is replaced with a M-0.1% Fe alloy under similar conditions using the given data
c) Qualitatively evaluate the effect of on cathodic reaction if this reaction is controlled by activation polarization at lower potential and becomes dominantly controlled by concentration polarization at high over potential. Justify your answer with schematic E-log I kinetics diagram
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


