Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A countercurrent absorption tower is used to strip SO2 (sulfur dioxide) from the gas produced by a smelter. The gas from the smelter contains 4,5
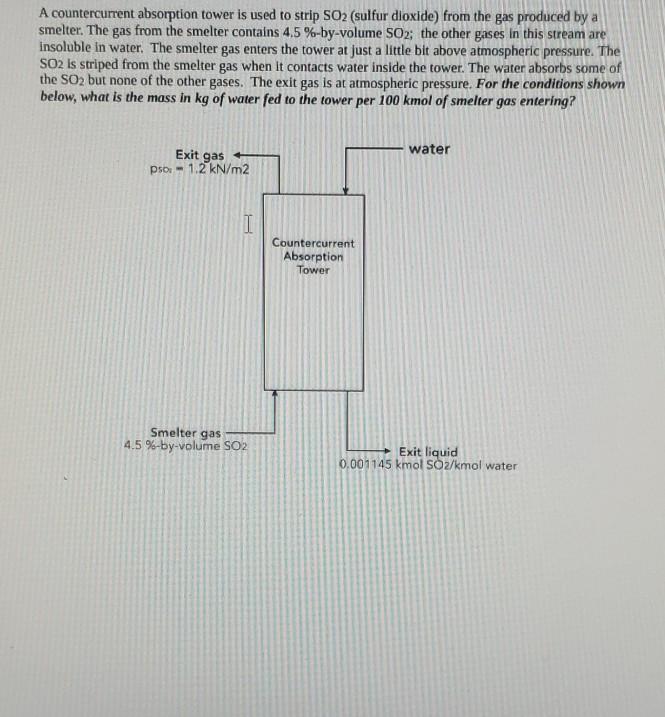
A countercurrent absorption tower is used to strip SO2 (sulfur dioxide) from the gas produced by a smelter. The gas from the smelter contains 4,5 %-by-volume SO2: the other gases in this stream are insoluble in water. The smelter gas enters the tower at just a little bit above atmospheric pressure. The SO2 is striped from the smelter gas when it contacts water inside the tower. The water absorbs some of the SO2 but none of the other gases. The exit gas is at atmospheric pressure. For the conditions shown below, what is the mass in kg of water fed to the tower per 100 kmol of smelter gas entering? Exit gas water pso - 1.2 kN/m2 I Countercurrent Absorption Tower Smelter gas 4.5 %-by-volume SO2 Exit liquid 0.001145 kmol SO2/kmol water
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


